Pan-tumor panel quality control products upgraded
In response to the needs of daily medical testing, the pan-tumor panel can very well help analyze and determine problems in routine testing. Effective and reasonable quality control can help analyze all human, machine, material, method, and environment.
We have upgraded our first large pan-tumor panel, which is mainly reflected in:
1. Introduce more pathologically significant mutations, fusions and copy number variations;
2. More diverse mutation types: missense mutations, nonsense mutations, synonymous mutations, splicing mutations, insertion mutations (short/long, repeated sequences), deletion mutations (short/long, repeated sequences), fusion mutations, copies number amplification;
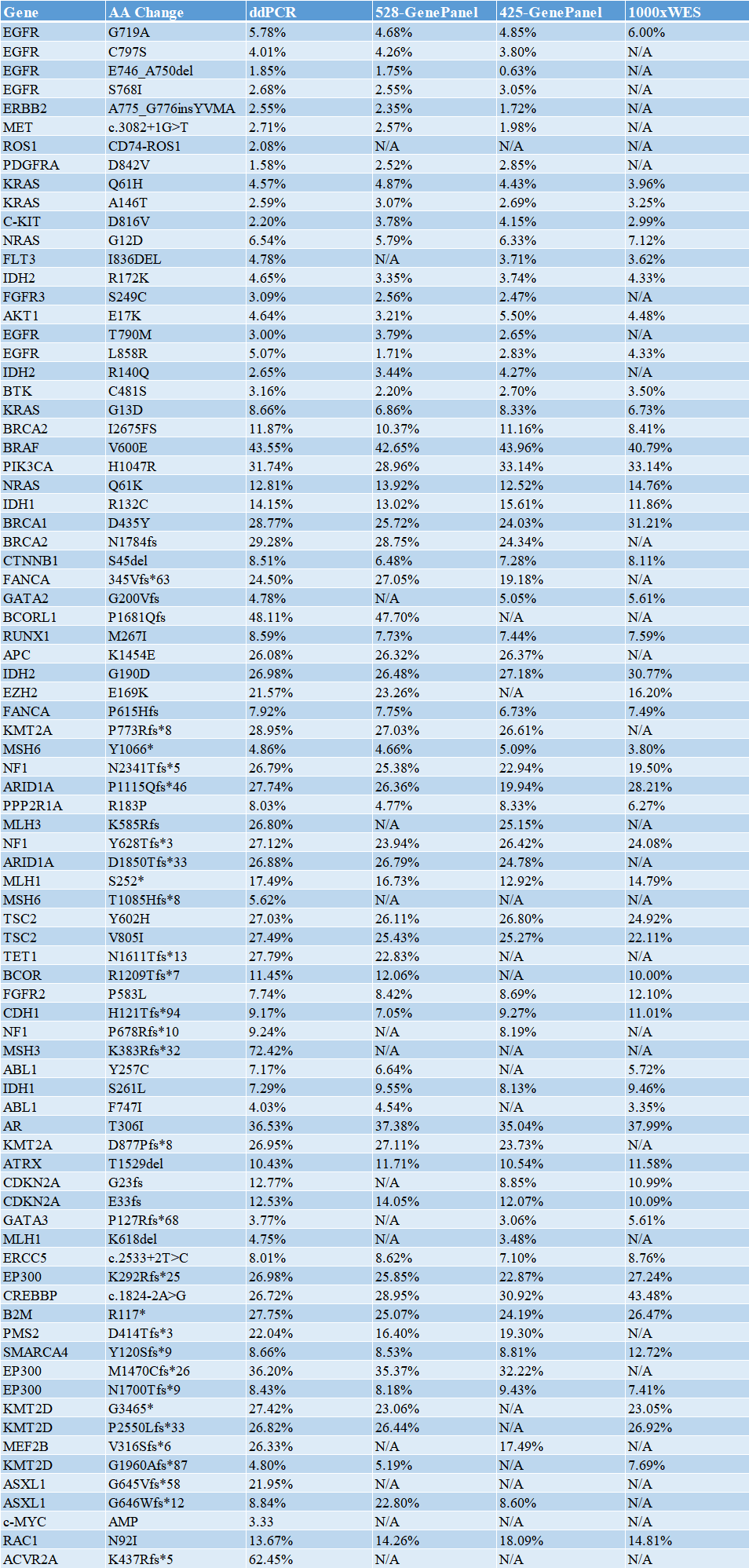
3. The calibration scheme is more scientific: 4 methods are used for calibration, namely 1000xWES, 3000x 425 Gene-Panel (friendship-tissue sample), 3000x 528 Gene-Panel (internally developed probe capture method), 82-site ddPCR (3 replicate wells);
4. The mutation frequency ranges from 1% to 100%, with a rich span;
5. Introduction of MSI-H (NGS method 92.31%, PCR method 100% MSI);
6. Introduce TMB score (Value=415.0 mutations/Mb);
7. Introduce MMR-related gene mutation detection;
RQP90043 Panel-Ref® gDNA All-in-One Reference Standard Plus
Partial data display (ddPCR calibration)
In order to give back to new and old customers, we will launch a limited-time free trial event in September. Interested teachers can contact us to try it out.

