

Lentiviral vector (LVs) is a virus vector system modified on the basis of HIV-1 virus, which can efficiently introduce the target gene (or RNAi) into animal and human primary cells or cell lines. The lentiviral vector genome is a positive-strand RNA. After its genome enters the cell, it is reversed into DNA by its own reverse transcriptase in the cytoplasm, forming a pre-DNA integration complex. After entering the nucleus, the DNA is integrated into the cell genome. The integrated DNA transcribes mRNA, returns to the cytoplasm, and expresses the target protein; or produces RNAi interference.
Lentiviral vector-mediated gene expression or RNAi interference is sustained and stable because the target gene is integrated into the host cell genome and divides as the cell genome divides. In addition, lentiviral vectors efficiently infect and integrate into non-dividing cells. The above characteristics make lentiviral vectors have distinct characteristics compared with other viral vectors, such as non-integrating adenoviral vectors, adeno-associated viral vectors with low integration rates, and traditional retroviral vectors that only integrate dividing cells. A large number of literature studies have shown that the tissues or cells of long-term expression of target genes mediated by lentiviral vectors include brain, liver, muscle, retina, hematopoietic stem cells, bone marrow mesenchymal stem cells, macrophages, etc.
The lentiviral vector does not express any HIV-1 protein, has low immunogenicity, has no cellular immune response at the injection site, and has a low humoral immune response, which does not affect the second injection of the viral vector.
Service Introduction
Lentivirus belongs to the Retroviridae family and is an RNA virus, such as human immunodeficiency virus (HIV), feline immunodeficiency virus (FIV), simian immunodeficiency virus (SIV), and bovine immunodeficiency virus. Since the research on HIV-1 is the most extensive and in-depth, and its biological characteristics are the best known, the lentiviral vector derived from HIV-1 has become a representative of it.
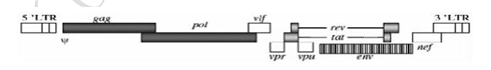
HIV-1 is a double-stranded RNA virus with a total of 9 genes, as shown in Figure 1. The three genes gag, pol, and env encode the basic structure of the virus, the tat and rev are regulatory genes, and the four auxiliary genes are vif, vpr, vpu, and nef. Among them, the gag gene encodes the core protein of the virus, including matrix protein, capsid protein, and nucleocapsid protein; the pol gene encodes the enzymes required for virus replication, such as reverse transcriptase, integrase, and protease, and the env gene encodes the viral package Membrane glycoproteins that determine the targeting of viruses to infected hosts. The protein encoded by rev regulates the expression levels of gag, pol, and env, and the protein encoded by tat participates in the control of RNA transcription. The proteins encoded by the four auxiliary genes are involved in the recognition and infection of host cells as virulence factors. Both ends are long terminal repeats (LTR), which contain cis-acting elements required for replication, such as packaging signal elements Ψ.
The first-generation lentiviral vector system is represented by the three-plasmid system, which consists of three kinds of plasmids: packaging plasmid, envelope plasmid and carrier plasmid. The packaging plasmid is that the 5' LTR of HIV-1 proviral genome is replaced by the early promoter of cytomegalovirus, and the 3' LTR is replaced by SV40 polyA sequence. The packaging components were constructed separately on two plasmids, one expressing gag and pol and the other expressing env. The vector plasmid carries the 5' LTR, the entire 5' untranslated region, and the rev response element (RRE). The envelope expression plasmid uses the vesicular stomatitis virus glycoprotein G gene (VSV-G) to replace the env gene of the original virus.
The second-generation lentiviral vector system is improved on the basis of the first generation, and all accessory genes of HIV (vif, vpr, vpu and nef genes) are deleted in the packaging plasmid. The removal of these accessory genes does not affect the titer and infectivity of the virus, while increasing the safety of the vector.
The third-generation lentiviral vector system replaces the original three-plasmid packaging system with four plasmids. Place the rev gene alone on a packaging plasmid. At the same time, two safety features are added: one is to construct a self-inactivated lentiviral vector, that is, to delete the 3'LTR of the U3 region, so that the vector loses the HIV-1 enhancer and promoter sequence, even if all viral proteins exist RNA cannot be transcribed; the second is to remove the tat gene and replace it with a heterologous promoter sequence, so that only 3 of the 9 lentiviral vectors in the original HIV-1 genome remain (gag, pol and rev). Therefore, the third-generation lentiviral vector system is safer.
Service Advantage
·Can effectively integrate exogenous genes into host chromosomes, and the expression level of exogenous genes is high and stable;
·Wide range of hosts, can infect dividing and non-dividing cells, can infect almost all types of cells, especially suitable for cells with low transfection efficiency of plasmid vectors;
·Wide range of applications, can be used in in vitro cell lines and living animal models, research genes and RNAi, etc.;
·High transduction efficiency, no transfection reagent required, and the probability of target gene integration into the host cell genome is greatly increased.
·Reqbio has rich experience in virus packaging, successfully packaged the required viruses for many customers, and completed the corresponding functional testing.
Service flow
1) Obtain the target gene sequence;
2) Construction of lentiviral vector;
3) Co-transfect the recombinant plasmid and the packaging plasmid to package the virus;
4) Virus purification and concentration;
5) Titer determination.
6) Infect the cells in the control group and the experimental group, and use antibiotics to screen to obtain stable monoclonal cell lines (optional).
Case Presentation
Service Description
1) The customer provides the sequence of the target gene, and if a template is provided, please inform the carrier, enzyme digestion stability point and other information;
2) If the experimental material is primary cells or specially cultured cells, please consult first;
3) The company provides a default virus titer of 1x107-1x108TU/ml, with a specification of 1ml;
4) The functional verification of the target protein will be charged additionally.
Ordering method
 Service Hotline:4008-750-250 Phone:180-6607-1954
Service Hotline:4008-750-250 Phone:180-6607-1954
 QQ:4008-750-250
QQ:4008-750-250  Mail:sales@reqbio.com
Mail:sales@reqbio.com