Standard products for deafness genetic testing
Background
Hearing impairment is one of the common birth defects in human beings. There are as many as 27.8 million disabled people with hearing impairment in my country, including about 137,000 hearing-impaired children aged 0-6, and 20,000-30,000 hearing-impaired children born every year. The incidence of congenital deafness in neonatal period is 1‰~1.86‰. Deafness is caused by genetic factors and/or environmental factors. The etiology of deafness is complex, and more than 60% are related to genetic factors. There are many genes related to hereditary deafness. The mutation carrier rate of deafness genes in the Chinese population is about 6.3%, and about 70% of the mutations come from four hotspot genes: GJB2, SLC26A4, mitochondrial DNA 12S rRNA and GJB3.
Screen genetic variation of genetic deafness for pre-pregnancy or pregnant couples, prenatal diagnosis for pregnant women can avoid the birth of deaf children; screening genetic variation of genetic deafness for newborns, early diagnosis and early treatment can effectively intervene in deafness The most important thing is that drug-induced deafness gene carriers and delayed-onset deafness gene carriers that cannot be detected by conventional physical hearing screening can be found through genetic variation screening, and drug-induced deafness can be avoided or delayed by health guidance. occurrence of deafness.
R & D status
With the development of genetic diagnosis technology, deafness gene detection technology has been widely used clinically. Genetic testing technologies that have been used clinically include gene chip technology, sequencing technology (NGS), multiple ligation probe amplification technology (MLPA), reverse dot hybridization technology (RDB), real-time fluorescence detection technology, time-of-flight mass spectrometry technology, Sanger sequencing, etc.
At present, 11 related detection reagent products have been approved for marketing.
Standard products for deafness genetic testing
In order to ensure the accuracy of deafness gene detection, the development of relevant kits cannot be separated from the escort of reference products. We have newly launched standard products for deafness genetic testing, which can be selected by manufacturers developing kits.
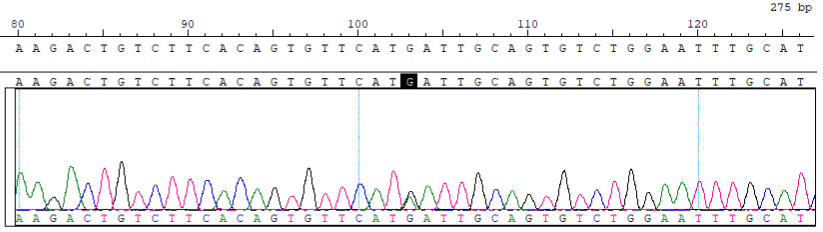
GJB2 c.176_191del Reference Standard RQPD0011
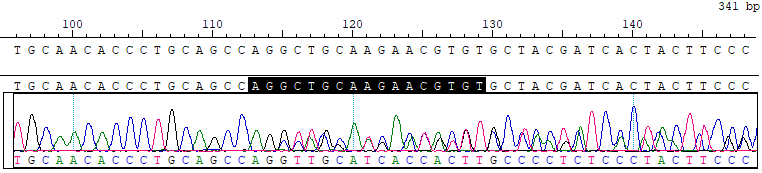
GJB2 c.235del Reference Standard RQPD0012
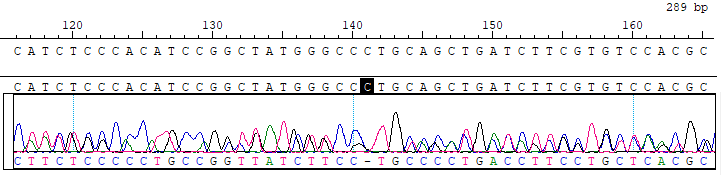
GJB2 c.358_360del Reference Standard RQPD0013
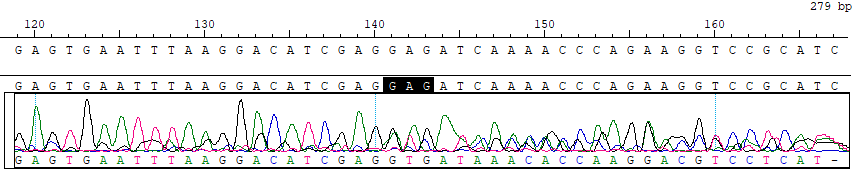
GJB2 c.585G>A Reference Standard RQPD0014