IL10 and its receptor cell screening model
Background
Interleukin-10 (IL-10), also known as human cytokine synthesis inhibitory factor (CSIF), is an anti-inflammatory cytokine. As an immunosuppressive cytokine, IL-10 is produced by a variety of cell types, including macrophages, monocytes, T cells, B cells, and keratinocytes. IL-10 has pleiotropic effects in immune regulation and inflammatory response. On the one hand, IL-10 can inhibit the production of inflammatory factors such as IFN-γ, IL-2, IL-3, Synthesis of TNFα and GM-CSF, and it can also inhibit the antigen-presenting ability of antigen-presenting cells; on the other hand, it also stimulates certain T cells and mast cells, and stimulates B cell maturation and antibody production. The receptor of IL-10 is a tetramer composed of two ligand-binding subunits IL-10R1 and two auxiliary signaling subunits IL-10R2. When IL-10 binds to IL-10R1, it activates IL-10R1. The IL-10 receptor composed of IL-10 and R2 leads to the phosphorylation of its downstream JAK1 and TYK2, and then participates in the regulation of the JAK-STAT signaling pathway.
Introduction to IL-10
IL-10 may be associated with a variety of diseases, and its overexpression may be closely related to systemic lupus erythematosus, tuberculosis, etc., while lack of IL-10 may be associated with inflammatory bowel disease, psoriasis, asthma, and rheumatoid arthritis. related to the occurrence. In the process of tumorigenesis, the role of IL-10 is controversial. In some cancers such as non-small cell lung cancer, diffuse large B-cell lymphoma, cervical cancer and oral cancer, the expression level of IL-10 is related to tumor A positive correlation occurred, while in colon cancer and prostate cancer patients, its expression was lower than that in the control population. On the one hand, since IL-10 is widely accepted as an immunosuppressive cytokine, IL-10 is thought to promote tumor immune escape by reducing antitumor immune responses in the tumor microenvironment; on the other hand, Ouyang W et al. There are at least three main anti-tumor mechanisms of IL-10: 1. Promote the proliferation and activation of CD8T+ cells, thereby enhancing the anti-tumor ability; 2. IL-10 inhibits the antigen presentation of myeloid cells and inhibits IFN-g-induced cells Factor production, especially inhibition of IL-12 expression can induce strong anti-tumor immunity; 3. Certain pro-inflammatory cytokines, such as IL-6 and IL-23, can promote tumor growth under chronic inflammation, and IL-10 can inhibit these Inhibition of cytokine production may be beneficial in certain cancers.
Current status of drug development
Perhaps it is precisely because of the complexity of IL-10's biological function and involvement in various disease mechanisms that the development of drugs targeting IL-10 and its signaling pathway has not been smooth for many years. For example, when Eli Lilly invested heavily in the acquisition of Armo Biosciences in 2018, The promising AM00100 (Pegilodecakin) in its pipeline has successively failed clinical trials for pancreatic cancer and non-small cell cancer. In addition, Merck's MK-1966, Y-Biologics' YBL010, Cytonus Therapeutics' CA-IL10, Ambrx's A number of drugs such as PEG-Cytokines and Biotest AG's BT-063 have also been discontinued, and nothing has happened.
Despite many setbacks, people have not given up on the research and development of this target drug, and finally achieved a certain breakthrough earlier this year. Applied Molecular Transport (AMT) first announced its oral IL-10 cytokine drug AMT in May this year. -101 achieved positive top-line results in a Phase 2 clinical trial of monotherapy in patients with chronic pouchitis, a refractory inflammatory bowel disease, and was recommended for advancement into a Phase 3 clinical study; thereafter, Top-line results from the Phase 2 MARKET trial of AMT-101 in patients with moderate-to-severe ulcerative colitis were also announced on July 6: Although patients receiving the combination of AMT-101 and adalimumab were significantly better than those receiving placebo There was little difference in the clinical response rate among patients who received adalimumab (33.3% vs 31.8%), but an analysis of outcomes for patients with shorter disease duration < 5 years showed a higher clinical response rate than patients who received adalimumab alone. Excellent (43.8% vs 15.4%), suggesting that combination therapy may be beneficial early in the course of the disease, and in terms of safety, AMT-101 is safe and well tolerated. We also look forward to further clinical data in the future, hoping to bring new choices and hope to patients.
In response to this love-hate target, we have developed a relevant cell screening model, which can be used to screen and test the function of drugs related to this target. The product information and related data are as follows.
IL10 Effector Reporter Cell RQP74158
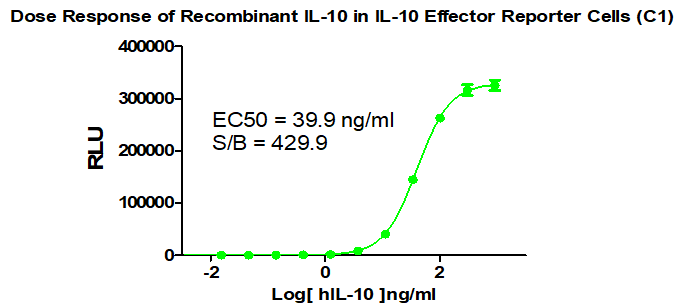
Figure 1. Dose Response of Recombinant IL-10 in IL-10 Effector Reporter Cells(C1).
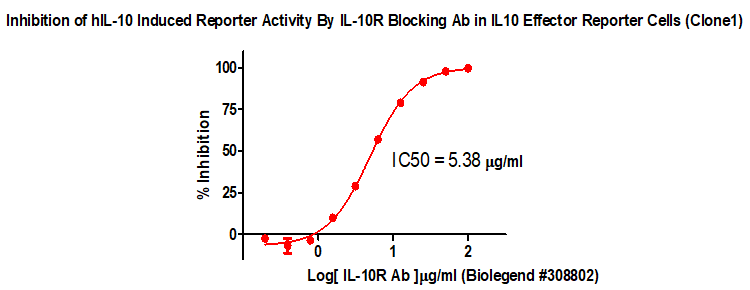
Figure 2. Inhibition of hIL-10 Induced Reporter Activity By IL-10R Blocking Ab in IL 10 Effector Reporter Cells(Clone 1).

