CD47/PD-(L)1 Dual Target Cell Screening Model
The ability of the PD-1 immune checkpoint to attenuate T-cell-mediated immune responses in patients whose tumors express its ligand, PD-L1, is well known. Although antitumor T-cell immune responses can be reactivated by treatment with blockade of PD-1 or PD-L1, immunosuppression remains prevalent in many tumors. In patients with these types of tumors, sufficient antitumor activity was not obtained by PD-1/PD-L1 inhibition alone. CD47, a cell surface molecule in the immunoglobulin superfamily, is another inhibitory immune checkpoint target that has been shown to be ubiquitously overexpressed on various malignant cells and is colloquially described as "don't eat me" "Signal conditioner. CD47 can bind to a variety of protein molecules, including signal regulatory protein alpha (SIRPα), signal regulatory protein gamma (SIRPγ), integrin and thrombospondin-1.5, etc. When CD47 binds to its receptor SIRPα (existing in various Myeloid cells), CD47 acts as an innate inhibitory checkpoint by blocking phagocytosis of tumor cells and downstream activation of innate immune responses, by inhibiting innate immune activation and tumor antigen presentation and initiating T cell responses , CD47 may allow tumor cells to evade innate as well as adaptive immune surveillance. In more than 50% of human cancers, the expression of MYC oncogene is deregulated and can simultaneously upregulate the expression of CD47 and PD-L1 on tumor cells, so the high expression of CD47 and PD-L1 in tumors often coexists.
In addition, CD47 overexpression has also been shown to be one of the resistance mechanisms to PD-1/PD-L1 therapy in preclinical models. Several preclinical studies have shown that blocking both CD47 and PD-L1 at the same time can significantly improve the effect of tumor immunotherapy. The combination of antibodies or the design of CD47&PD-L1 double antibody inhibits tumor growth in mouse models significantly better than a single target. spot antibodies. Therefore, the development of therapeutic regimens that simultaneously block CD47 and PD-1/PD-L1 has become a hot research direction for many pharmaceutical companies.
Current status of drug development
Several CD47 drug candidates have been combined with PD-1/PD-L1 inhibitors in the clinical stage.
Furthermore, with the development of double antibody technology in recent years, many developers have also designed and developed CD47&PD-(L)1 bispecific antibodies, hoping to reduce side effects by reducing the affinity of a single target, while improving synergistic enrichment. It specifically acts on the tumor microenvironment to enhance drug efficacy. Taking Pfizer's PF-07257876 as an example, its antibody is designed to have a low affinity for CD47 and a high affinity for PD-L1. The high binding of -L1 allows it to selectively bind to PD-L1-expressing cells in the tumor microenvironment, further reducing binding to erythrocytes. At present, a number of CD47/PD-(L)1 dual antibodies have entered the clinical stage at home and abroad
CD47/PD-(L)1 Dual Target Cell Screening Model
For the development of CD47/PD-(L)1 antibody drugs, Kebai Bio has specially developed a CD47/PD-(L)1 dual-target cell screening model, which can functionally measure and evaluate CD47/PD-(L)1 from the cellular level. (L)1 The efficacy of the combination drug and bispecific antibody, product information and related data are as follows:
SIRPɑ/PD-1 Dual Effector Reporter Cell CBP74154
CD47/PD-L1 Dual Target Cell CBP74155
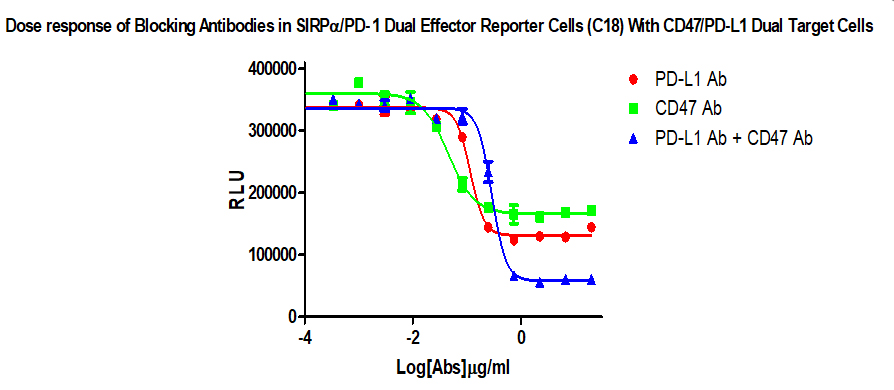
Figure 1. Dose response of Blocking Antibodies in SIRPα/PD-1 Dual Effector Reporter Cells (C18) With CD47/PD-L1 Dual Target Cells.

