Fourth-generation ALK kinase inhibitor screening model
background
Chromosomal rearrangements of the anaplastic lymphoma kinase (ALK) gene produce oncogenic fusion proteins that are present in approximately 3% to 5% of non-small cell lung cancer patients and exhibit abnormal dimerization or oligomerization, resulting in constitutive ALK activation that drives tumorigenesis.
Research and development status
In preclinical NSCLC models, the fusion of ALK and EML4 genes to form EML4-ALK was found to be highly oncogenic, while clinically, targeting the EML4-ALK kinase domain with small-molecule ALK inhibitors has clinically significant clinical effects in ALK+ NSCLC patients benefit. To date, three generations of ALK inhibitor drugs have been approved, including the first generation (crizotinib), the second generation (alectinib, bagatinib, and cititinib) and the third generation (lorlatinib) Ni) and so on. However, due to the emergence of some mutations in ALK during the medication process, which interfere with drug binding, the therapeutic effects obtained with these drugs are often not sustained. Crizotinib was first approved for patients with ALK+ NSCLC in 2011 and achieved an objective response rate of 74% in first-line therapy; however, crizotinib treatment can lead to resistance mutations in the active site of ALK, such as G1269A , C1156Y, L1196M allele amino acid mutations, which limit the durability of its efficacy. The second-generation ALK inhibitors (alectinib, bagatinib, cictinib) have been approved for use in patients with ALK+ non-small cell lung cancer. Although they can overcome the resistance mutations caused by the use of some first-generation inhibitors to a certain extent, the Also susceptible to some specific ALK resistance mutations, such as G1202R (found in 33% to 37% of relapsed patients), I1171 mutation (found in 24%-26% of patients), and L1196M mutation (17%–22%) found in patients), etc. The third-generation ALK inhibitor lorlatinib is approved for patients with ALK+ NSCLC who have been previously treated with crizotinib and at least one other ALK inhibitor, or after first-line therapy with alectinib or cetirtinib L1196M (38%), G1202R (28%), D1203N (24%), L1196M (38%), G1202R (28%), D1203N (24%) were detected in 76% of plasma samples from patients with disease progression after lorlatinib treatment, although it was shown to be sensitive to the G1202R site mutation. %), F1174C/L (14%), and I1171X (14%) alleles, these mutations often exist in the form of compound mutations, which are statistically found in 35% to 48% of treated patients. This suggests that the drug is not sensitive to compound mutations, and multiple ALK compound mutations are its main drug resistance mechanism, among which it has been confirmed that the G1202R/L1196M mutation combination is resistant to all marketed ALK inhibitors.
In order to overcome the above-mentioned various resistance mutations of existing ALK inhibitors, especially compound resistance mutations, the fourth-generation ALK inhibitors are also under continuous development, among which TPX-0131 and NUV-655 are the most prominent. TPX-0131 is a compact macrocyclic structure that facilitates insertion into the adenosine-binding pocket, with clear boundaries to L1196 and G1202, thus facilitating binding to these site mutations to exert its inhibitory effect. Preclinical data show that TPX0131 is less sensitive to ALK wild-type, ALK common mutations, and various compound mutations, except for I1171N/S/T and G1269S that are less sensitive than ceritinib, alectinib, and lorlatinib All are better than the first three generations of ALK inhibitors; the drug is currently in the Phase 1/2 FORGE-1 clinical trial in ALK+ advanced non-small cell lung cancer (NSCLC) patients (NCT04849273). NUV-655 is a fourth-generation tyrosine kinase inhibitor targeting ALK/ROS1, which maintained a series of resistance mutations in cells generated by first-, second- and third-generation ALK inhibitors. Good activity, including G1202R single mutation or compound mutation G1202R/L1196M, G1202R/G1269A, G1202R/L1198F, etc., the drug is about to enter the clinical trial stage.
In order to help the development of a new generation of ALK inhibitors, we have developed a series of ALK wild and mutant cell lines, especially those containing multiple combinations of compound mutations, which can be used for in vitro activity screening of inhibitors against these drug-resistant mutation combinations.
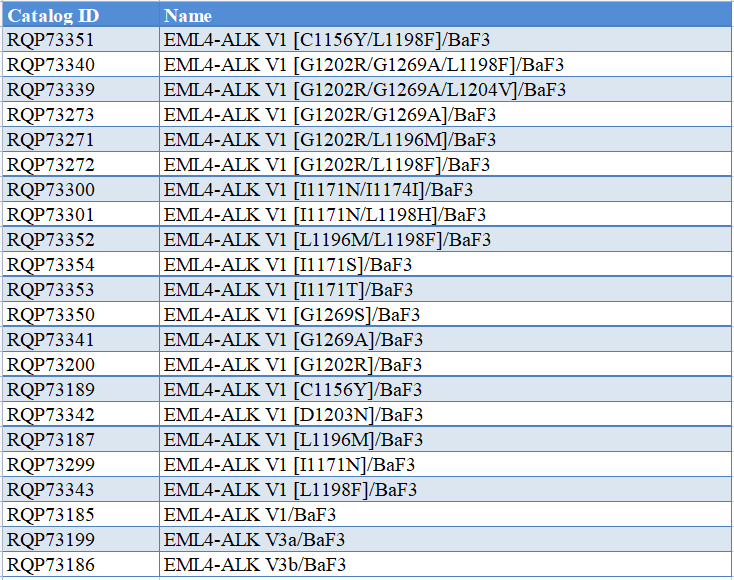
Product List
Part of the data graph display
1.EML4-ALK V1 [C1156Y/L1198F]/BaF3 RQP73351

Figure 1. CTG Proliferation Assay of BaF3 EML4-ALK V1 [C1156Y/L1198F] (C1).
2.EML4-ALK V1 [G1202R/G1269A/L1198F]/BaF3 RQP73340

Figure 2. CTG Proliferation Assay of BaF3 EML4-ALK V1 [G1202R/G1269A/L1198F] (C3).
3.EML4-ALK V1 [G1202R/G1269A/L1204V]/BaF3 RQP73339

Figure 3. CTG Proliferation Assay of BaF3 EML4-ALK V1 [G1202R/G1269A/L1204V] (C1).
4.EML4-ALK V1 [G1202R/G1269A]/BaF3 RQP73273

Figure 4. CTG Proliferation Assay of EML4-ALK V1 [G1202R/G1269A] /BaF3.
5.EML4-ALK V1 [G1202R/L1196M]/BaF3 RQP73271

Figure 5. CTG Proliferation Assay of BaF3 EML4-ALK V1 [G1202R/L1196M] (C1).
6.EML4-ALK V1 [G1202R/L1198F]/BaF3 RQP73272

Figure 6. CTG Proliferation Assay of BaF3 EML4-ALK V1 [G1202R/L1198F] (C1).
7.EML4-ALK V1 [I1171N/I1174I]/BaF3 RQP73300

Figure 7. CTG Proliferation Assay of BaF3 EML4-ALK V1 I1171N/F1174I (C10).
8.EML4-ALK V1 [I1171N/L1198H]/BaF3 RQP73301

Figure 8. CTG Proliferation Assay of BaF3 EML4-ALK V1 I1171N/L1198H (C1).
9.EML4-ALK V1 [L1196M/L1198F]/BaF3 RQP73352

Figure 9. CTG Proliferation Assay of BaF3 EML4-ALK V1 [L1196M/L1198F] (C1).
10.EML4-ALK V1 [I1171S]/BaF3 RQP73354

Figure 10. CTG Proliferation Assay of BaF3 EML4-ALK V1 [l1171S] (C1).
11.EML4-ALK V1 [I1171T]/BaF3 RQP73353

Figure 11. CTG Proliferation Assay of BaF3 EML4-ALK V1 [l1171T] (C1).
12.EML4-ALK V1 [G1269S]/BaF3 RQP73350

Figure 12. CTG Proliferation Assay of BaF3 EML4-ALK V1 [G1269S] (C1).
13.EML4-ALK V1 [G1269A]/BaF3 RQP73341

Figure 13. CTG Proliferation Assay of BaF3 EML4-ALK V1 [G1269A] (C1).
14.EML4-ALK V1 [G1202R]/BaF3 RQP73200

Figure 14. Anti-proliferation assay of three reference compounds on the EML4-ALK v1 [G1202R]/BaF3 Stable Cell Line.
15.EML4-ALK V1 [C1156Y]/BaF3 RQP73189

Figure 15. Anti-proliferation assay of three reference compounds on the EML4-ALK V1 C1156Y/BaF3 Stable Cell Line.
16.EML4-ALK V1 [D1203N]/BaF3 RQP73342

Figure 16. CTG Proliferation Assay of BaF3 EML4-ALK V1 [D1203N] (C1).
17.EML4-ALK V1 [L1196M]/BaF3 RQP73187

Figure 17. Anti-proliferation assay of three reference compounds on the EML4-ALK V1 L1196M/BaF3 Stable Cell Line.
18.EML4-ALK V1 [I1171N]/BaF3 RQP73299

Figure 18. CTG Proliferation Assay of BaF3 EML4-ALK V1 I1171N (C3).
19.EML4-ALK V1 [L1198F]/BaF3 RQP73343

Figure 19. CTG Proliferation Assay of BaF3 EML4-ALK V1 [L 1198F] (C1).
20.EML4-ALK V1/BaF3 RQP73185

Figure 20. Anti-proliferation assay of three reference compounds on the EML4-ALK V1/BaF3 Stable Cell Line.
21.EML4-ALK V3a/BaF3 RQP73199

Figure 21. CTG Proliferation Assay of BaF3 EML4-ALK V3a (C1).

