Iterative breakthrough innovation of old targets: Her2 target cell model
The ASCO meeting in 2022 is about to be held. Judging from some of the published abstracts, the Her2 target will once again become one of the focuses of this meeting. AstraZeneca/Daiichi Sankyo, Spectrum, BeiGene, Corning & Jereh, Kelun Pharmaceutical, Rongchang Bio, Lepu Bio and other pharmaceutical companies use different technical routes to treat Her2-positive tumors, and the clinical data released one after another are all It has good curative effect and deserves special attention.
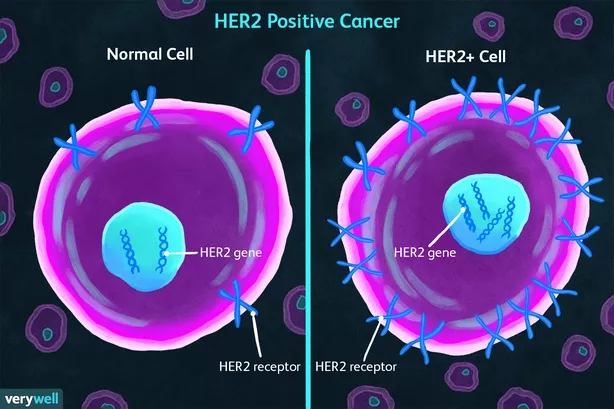
Fig 1. Her2 Positive Cancer
Introduction to HER2
Her2 is an oncogene that was discovered earlier. It was discovered by Schechter et al. in 1984. In the past 40 years, the mechanism of action, drugs and new therapies of this target have been continuously discovered and innovated, and great progress has been made. It is also a drug target with great potential point. The HER protein family consists of four highly homologous protein members, EGFR (ERBB1/HER1), Her2 (ERBB2), HER3 (ERBB3) and HER4 (ERBB4), which control cell growth, survival, differentiation and migration. The Her2 ectodomain has no known ligands and is activated by forming homo- or heterodimers. Dimerization leads to phosphorylation of tyrosine kinase residues in the cytoplasmic domain, which activate signaling pathways such as phosphatidylinositol triphosphate kinase (PI3K) and mitogen-activated protein kinase (MAPK), leading to cell cycle progression, cellular differentiation and proliferation. Therefore, Her2 overexpression/mutation is closely related to the degree of malignancy of many epithelial cell cancers. In recent years, Her2 antibody drugs have made new breakthroughs in tumor treatment with the advantages of high specificity, efficacy and safety.
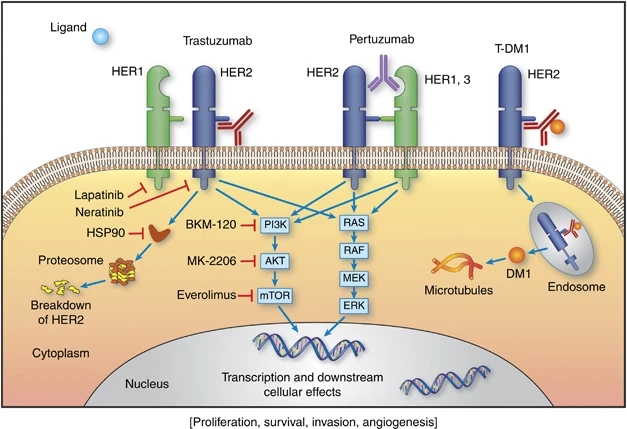
There are two main pathogenic mechanisms of Her2 currently found in clinical practice:
1. The high protein expression caused by the amplification of the Her2 gene mainly occurs in about 20%-25% of breast cancers (called Her2-positive breast cancers) and some gastric cancers, gastroesophageal junction cancers, biliary tract cancers, colorectal cancers, and non-small cell carcinomas. Lung cancer and bladder cancer, etc.
2. Her2 mutations, including Her2 point mutation and Her insertion mutation, the most important of which is Her2 exon20 insertion mutation, which is common in non-small cell lung cancer (NSCLC), accounting for about 2.27%, Her2 A775_G776insYVMA, G776delinsVC and P780_Y781insGSP these three kinds Insertional mutations are the most common.
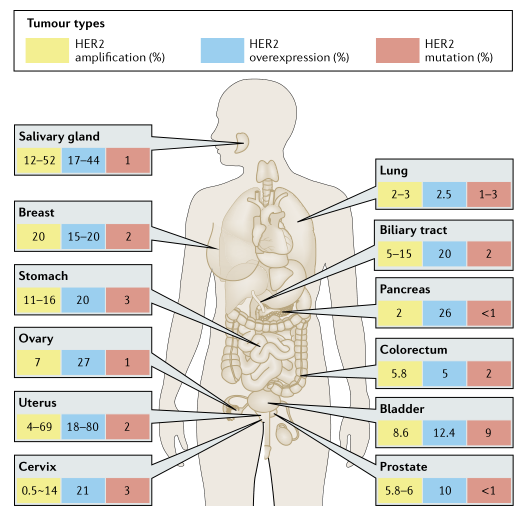
Current status of HER2-targeted drugs
At present, there are several drugs targeting Her2 on the market, and the global sales scale in 2021 will exceed 12.6 billion US dollars. Among them, trastuzumab (trastuzumab) and pertuzumab (pertuzumab) currently have the highest sales volume, and Her2 ADC drugs such as AstraZeneca/Daisankyo's trastuzumab emtansine (trastuzumab) have the most significant growth trend .
From the perspective of drugs under research, foreign Roche, takeda, NantCell, 3SBio, Biocad, Chugai Pharmaceutical, EirGenix, Immunwork, Marker Therapeutics, Refuge Biotechnologie all have drugs in the clinical development stage; domestic Kelun Pharmaceutical, Qilu Pharmaceutical, Corning Jereh, Hisun, Hengrui Medicine, Henlius, BeiGene, Kangyuan, Chia Tai Tianqing, Lepu Bio, etc. are also making arrangements in this target. Among them, it is worth noting that BeiGene's ZW25 double antibody, Kelun Pharmaceutical's A166ADC drug, Corning Jereh's KN026 double antibody, and Lepu Bio's MRG002 ADC drug. In addition, it is particularly worthy of attention to the new development of Her2 exon20 insertion drugs. Takeda Pharmaceutical's mobocertinib is a potent small molecule tyrosine kinase inhibitor of EGFR and Her2 exon20 insertion mutation (dual target), filling the Her2 exon 20 insertion mutation small molecule Treatment blank.
With technological progress and research innovation, new drugs with different characteristics such as Her2 ADC, double antibody, and CAR-T are still emerging. The field of Her2 target therapy continues to expand. In addition to the dominant field of Her2-positive breast cancer, other indications such as gastric cancer, gastroesophageal junction cancer, and urothelial cancer have also been approved. Therefore, the iterative innovation of Her2 drug research and clinical treatment is far from reaching the bottleneck, and breakthroughs and changes will continue to move forward.
HER2 target cell model
In response to the needs of Her2 target mechanism research and new drug development, Kebai Bio has launched a series of Her2 target drug screening cell models. Some data are shown below. Welcome to inquire.
RQP73110 Her2 WT/BaF3
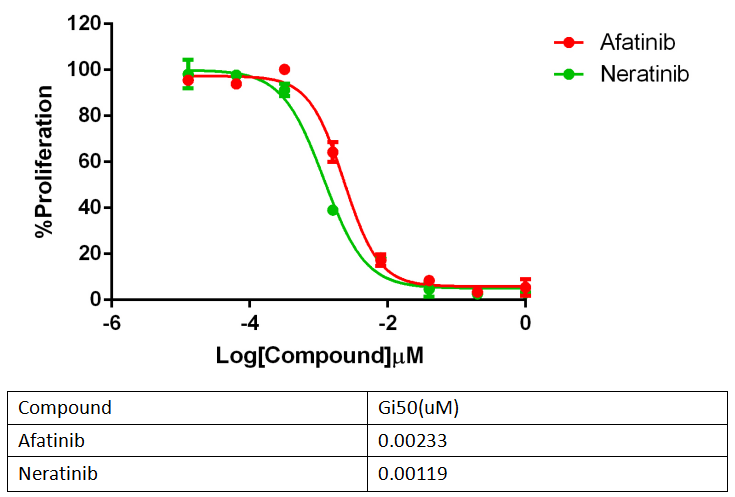
Fig 4. Anti-proliferation assay of two reference compounds on the Ba/F3_HER2 WT Stable Cell line.
RQP73319 Her2 L755S/BaF3
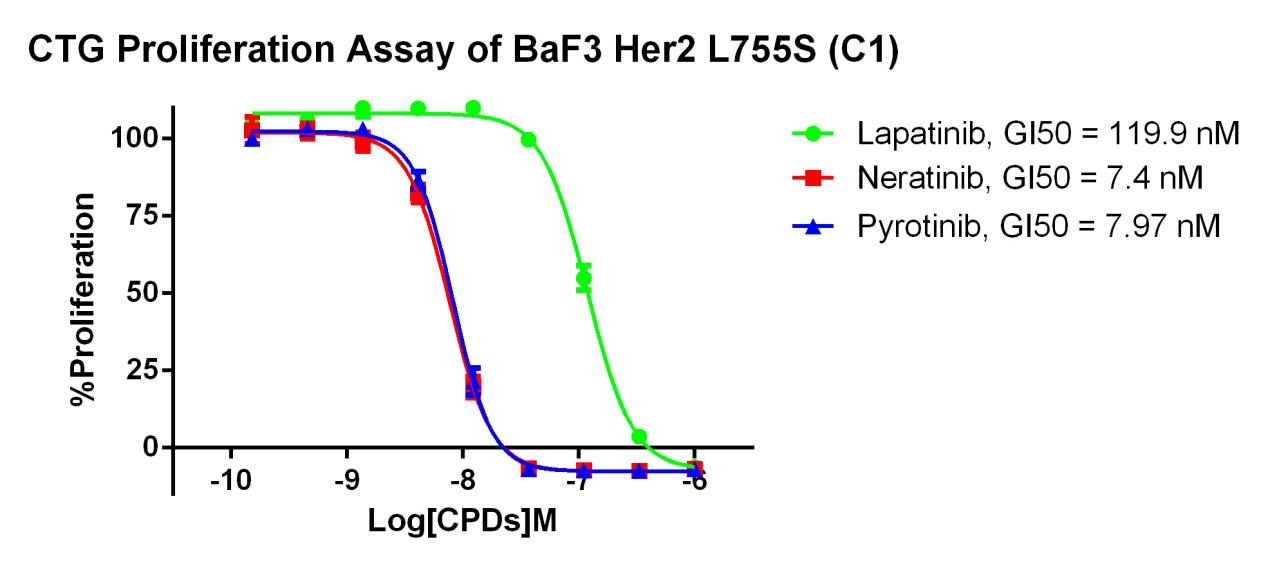
Fig 5. CTG Proliferation Assay of BaF3 Her2 L755S (C1).
RQP73184 Her2 A775_G776insYVMA/BaF3
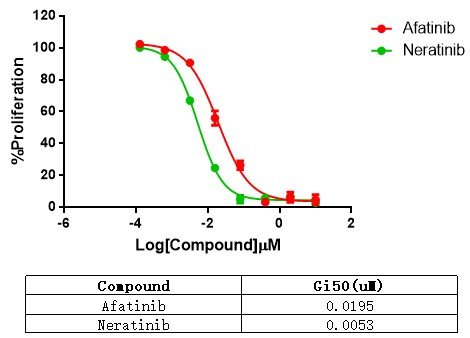
Fig 6. Anti-proliferation assay of two reference compounds on the Her2 A775_G776insYVMA/BaF3 Stable Cell Line.
RQP73244 Her2 P780_Y781insGSP/BaF3
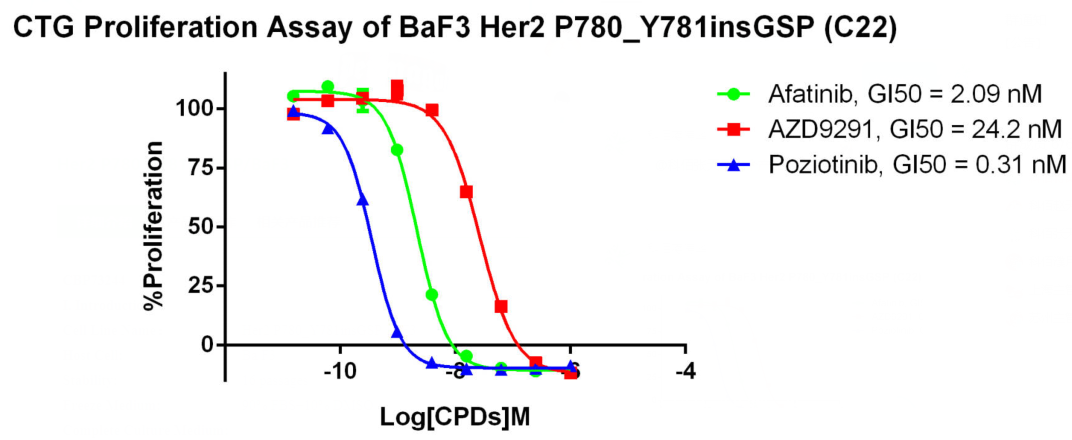
Fig 7. CTG Proliferation Assay of BaF3 Her2 P780_ Y781insGSP (C22).
RQP75003 MC38-HER2
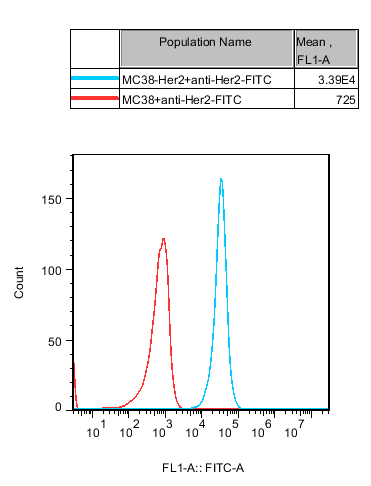
Fig 8. Recombinant MC38-Her2 stably expressing Her2.
RQP75004 CT26-HER2
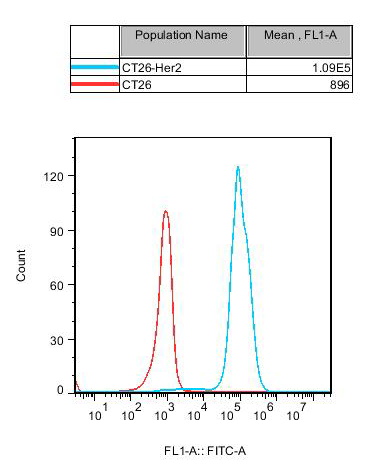
Fig 9. Recombinant CT26-Her2 stably expressing Her2.

