【Target Model】Drug Development of AKT Gene
The serine/threonine kinase AKT, also known as protein kinase B (PKB), is a key component of the phosphatidylinositol 3-kinase (PI3K) intracellular pathway and plays a key role in regulating cell proliferation, survival, and metabolism. The three AKT isoforms (AKT1, AKT2, and AKT3) are encoded by distinct genes with high sequence homology and display conserved protein structures. In cancer cells, AKT1 is involved in proliferation and growth, promotes tumorigenesis and inhibits apoptosis, while AKT2 regulates cytoskeletal dynamics, favoring invasion and metastasis, and the role of AKT3 hyperactivation in cancer remains controversial.
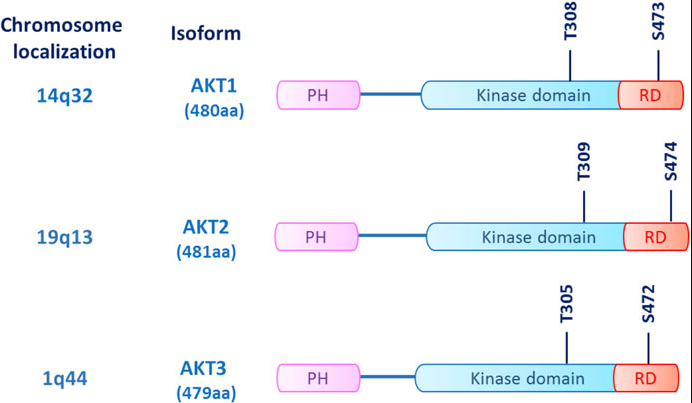
Fig 1. AKT structure: The three AKT isoforms (AKT1/2/3) are kinases with a common structure consisting of an N-terminal homology (PH) domain, a kinase domain, and a C-terminal regulatory domain (RD). )composition.
In intracellular signaling, AKT recruitment is primarily dependent on the generation of phosphatidylinositol triphosphate (PIP3) from PI3K, which is produced by receptor-coupled tyrosine kinases (RTKs), RAS-associated GTPases, and heterotrimeric G proteins activation. Interactions between the PI3K regulatory subunit (p85) and upstream effectors via the SRC homology 2 (SH2) domain determine the release and activation of the p110 catalytic subunit, which converts phosphatidylinositol-bisphosphate ( PIP2) into PIP3. PIP3 recruits its substrates, including AKT and phosphoinositide-dependent kinase 1 (PDK1), to the plasma membrane. Here, AKT undergoes dual phosphorylation, one on the kinase domain (T308, T309 and T305 for AKT1, 2 and 3, respectively) and the other on the regulatory domain (S473, S474 and T305 for AKT1, 2 and 3, respectively). S472), respectively, are activated by mToR complex 2 (mTORC2), resulting in its full activation. Once activated, AKT phosphorylates its downstream targets, including tuberous sclerosis complex 2 (TSC2), glycogen synthase kinase 3β (GSK3β), and forkhead kinase transcription factor (FOXO), ultimately promoting cell proliferation, metabolism, and survival .
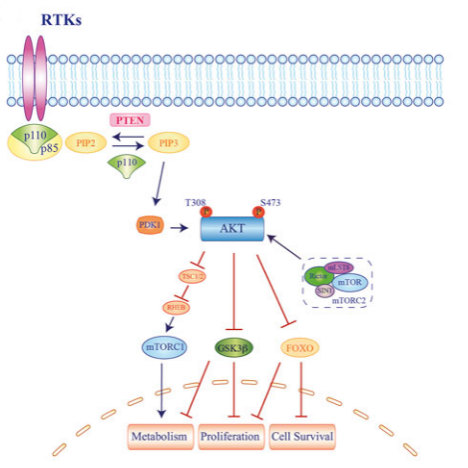
Fig 2. Molecular mechanism of AKT-activated signaling
Drug development for AKT
The effect of AKT on the occurrence, development, activation and inhibition of apoptosis of tumors is mainly through the activation and inhibition of downstream target substrates, and at the same time, it can also activate and inactivate a variety of transcription factors. The development of inhibitors is more difficult. Today, inhibitors against AKT are mainly divided into four categories.
• The first class consists of competitive inhibitors of the ATP binding site on the kinase domain. Includes drugs specifically targeting AKT isoforms, including CCT128930 targeting AKT2 and BAY-1125976 targeting AKT1 and AKT2. Pan-AKT kinase inhibitors include auresertib, AZD5363, GDC-0068, GSK690693 and AT7867.
• The second class is allosteric inhibitors of the AKT kinase domain, the most famous example of which is MK-2206.
• The third class are lipid-based inhibitors that prevent PI3K from generating PIP3 from PIP2, thereby indirectly inhibiting the activation of all AKT isoforms. Includes PX-866 and perifosine. These drugs also inhibit the NFkB pathway and c-Jun.
• A fourth class includes drugs that interact with the PH domain of AKT, thereby preventing AKT from localizing to the cell membrane where it is normally activated. Notable examples in this category include Trixilibine and PX-316. Since the PH domains of the three isoforms of AKT share less sequence identity compared to the kinase domain, drugs aimed at targeting the PH domain may be the future development of highly selective isoform-specific inhibitors best chance.
In addition, AKT has a low mutation rate in tumors, mainly at E17K in the PH domain, which leads to increased ATK activity by promoting the constitutive localization of AKT to the plasma membrane.
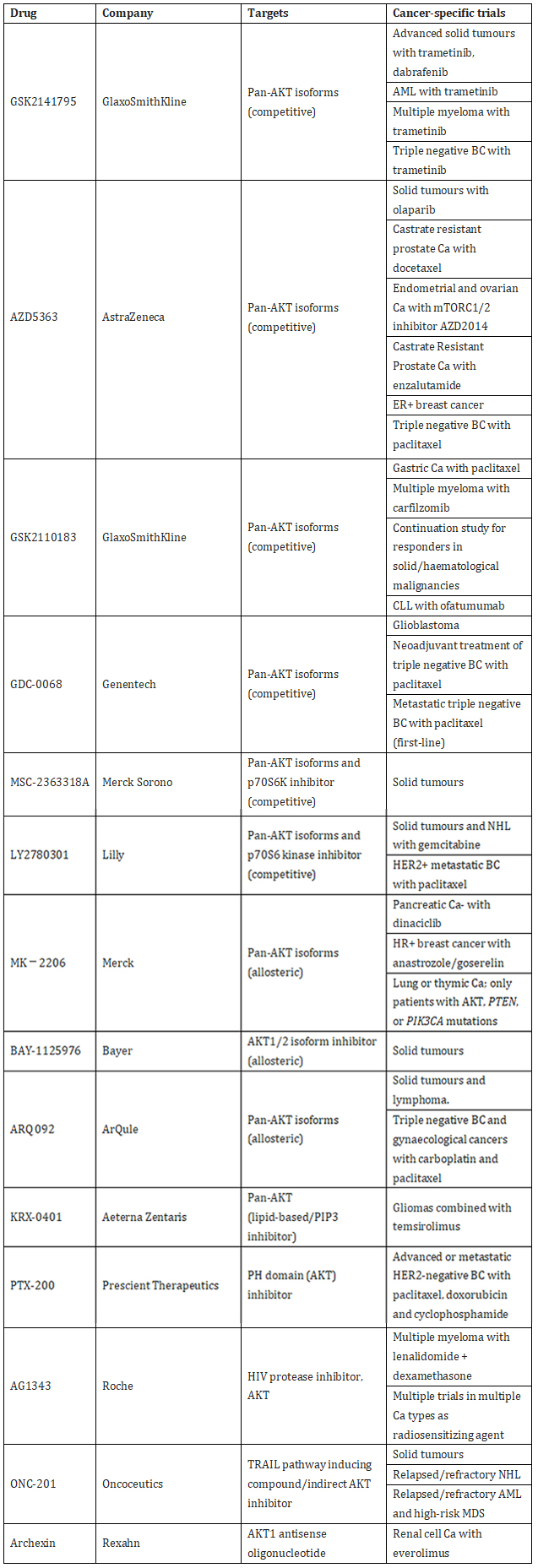
Table 1. Common AKT inhibitors
For the AKT target, we developed a cell-based assay model based on BaF3 mother cells, which can be used for in vitro and in vivo drug activity detection and drug QC release.
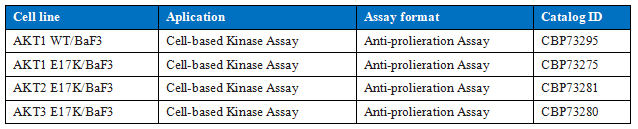
Table 2. Cell-based AKT assay model cell lines
Data Display
AKT1 WT/BaF3 RQP73295
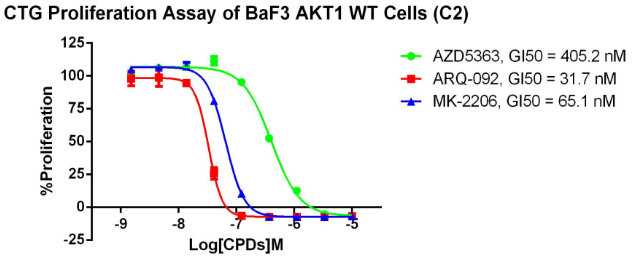
Fig 3. CTG anti-proliferation assay of BaF3 AKT1 WT.
AKT1 E17K/BaF3 RQP73275
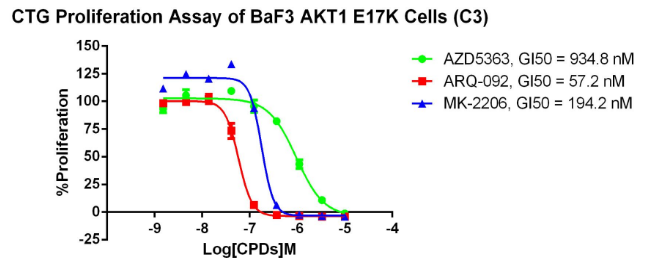
Fig 4. CTG anti-proliferation assay of BaF3 AKT1 E17K.
AKT2 E17K/BaF3 RQP73281
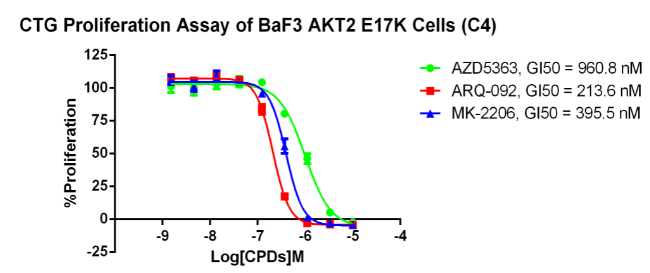
Fig 5. CTG anti-proliferation assay of BaF3 AKT2 E17K.
AKT2 E17K/BaF3 RQP73280
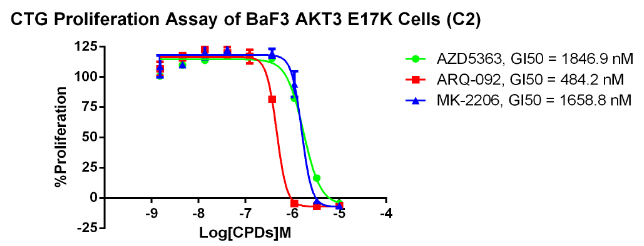
Fig 6. CTG anti-proliferation assay of BaF3 AKT3 E17K.
Drug Target Model
The reporter gene cell model can well reflect the molecular mechanism of action, and has less variability and better operability. Quality control and batch release are all important.
Kebai Biotechnology attaches great importance to R&D and innovation. With its cell function transformation technology, high precision and flexible gene editing tool platform, it provides drug detection cell models including kinases, GPCRs, immunotherapy, drug resistance and other disease targets. At present, it has covered more than 400 spot cell models, and provides high-quality Cell-base biological assay services.

