KRAS G12C drug-resistant mutant drug screening cell model
RAS protein is a guanosine triphosphatase (GTPase), including three subtypes of KRAS, HRAS, and NRAS. Among human tumors, KRAS mutations have the highest frequency, occurring in about 85% of cancers.
KRAS proteins can cycle between an activated guanosine triphosphate (GTP)-bound state and an inactive guanosine diphosphate (GDP)-bound state to function as a molecular switch. KRAS mutations mainly occur at amino acids 12, 13 and 61, disrupting GAP (GTPase activating protein)-mediated GTP hydrolysis, making KRAS mutant proteins prone to GTP-bound activation. The KRAS mutant protein has a significantly increased affinity for GTP, which leads to the continuous activation of downstream signal transduction pathways, including the activation of RAF-MEK-ERK, PI3K-AKT-mTOR, NF-KB and other signaling pathways, which promotes cell proliferation and metastasis, and promotes tumorigenesis and development .

Fig 1. KRAS mutation
KRAS target drug development and drug resistance
In the past 40 years, due to the strong affinity of KRAS mutant protein with GTP, high intracellular GTP concentration, and the lack of obvious hydrophobic pockets on the surface of KRAS protein, KRAS mutation was considered to be an "undruggable" target until Shokat et al. Nature reported the first KRAS G12C covalent inhibitor compound 12. In 2019, Amgen and Mirati Therapeutics developed two KRAS G12C inhibitors, AMG510 and MRTX-849, which entered clinical trials. The most common KRAS mutation is the mutation of the 12th glycine, and the mutant forms include KRAS G12C, KRAS G12D and KRAS G12V.
KRAS G12C covalent inhibitors such as AMG510 and MRTX849 developed in recent years can specifically covalently bind to inactive forms of KRAS G12C, and inhibit the activity of KRAS G12C by affecting the transition of KRAS G12C protein between inactive and active forms, thereby achieving anti-tumor effects. the goal of.
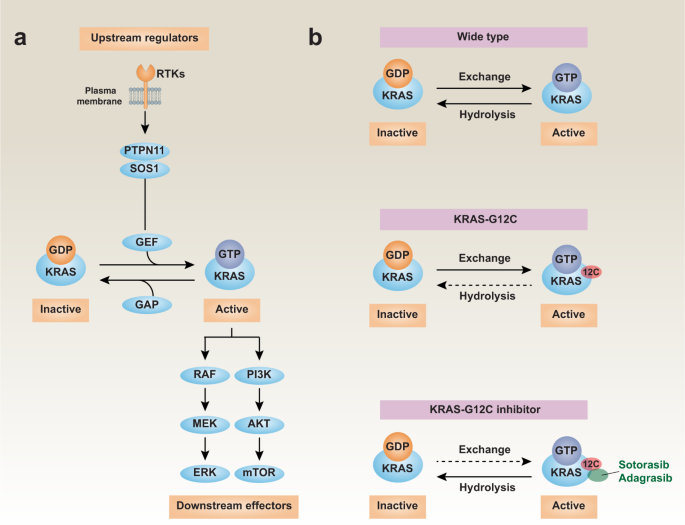
Fig 2. The mechanism of action of KRAS G12C covalent inhibitor
Amgen's AMG510 (sotorasib) has been approved for marketing in 2021, while Mirati Therapeutics' adagrasib (MRTX849) has been granted breakthrough therapy designation for patients with KRAS G12C-mutant NSCLC. Clinical benefit has been observed in many cancer patients treated with KRAS G12C inhibitors, but most patients eventually develop acquired resistance to monotherapy, therefore addressing both intrinsic and acquired resistance to KRAS G12C inhibitors mechanism, is critical to maximizing its therapeutic potential.
At present, the mechanism of resistance to KRAS G12C inhibitors and methods for overcoming resistance are being intensively studied. The mechanisms of resistance that have been discovered include:
● KRAS acquired resistance mutations (G12D/R/V/W, G13D, Q61H, R68S, H95D/Q/R, Y96C, and G12C amplification)
● Bypass activation (MET amplification; NRAS, BRAF, MAP2K1, and RET activating mutations; ALK, RET, BRAF, RAF1, and FGFR3 fusions; NF1 and PTEN inactivating mutations)
● Histopathological transformation (transformation of adenocarcinoma to squamous cell carcinoma)
Among them, KRAS acquired drug resistance mutations, especially KRAS G12C double mutations, have a high proportion, which is a hot direction for future mechanism research and new drug development.
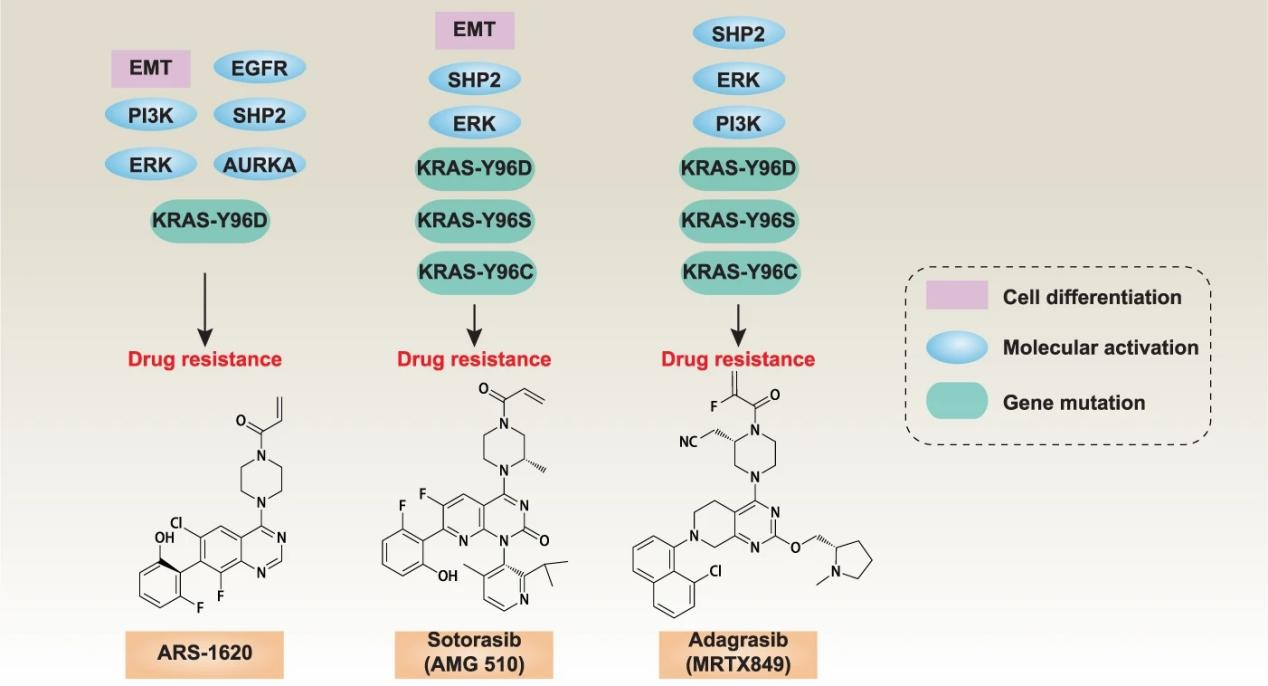
Currently, there is no drug for KRAS G12C drug resistance double mutation into the clinic, and many drugs are in the preclinical research stage. Among them, the new KRAS inhibitor tricomplex of Revolution Medicines has attracted more attention. Due to its different mechanism of action from existing KRAS G12C covalent inhibitors, it is still effective against KRAS G12C mutation and KRAS G12C resistance double mutation.
KRAS G12C Double Mutation Drug Screening Cell Model
In response to the needs of KRAS G12C drug resistance mutation mechanism research and new drug development, Kebai Bio has launched a series of KRAS G12C double mutation drug screening cell models. Some of the data are shown below.
KRAS G12C-H95D/BaF3 RQP73338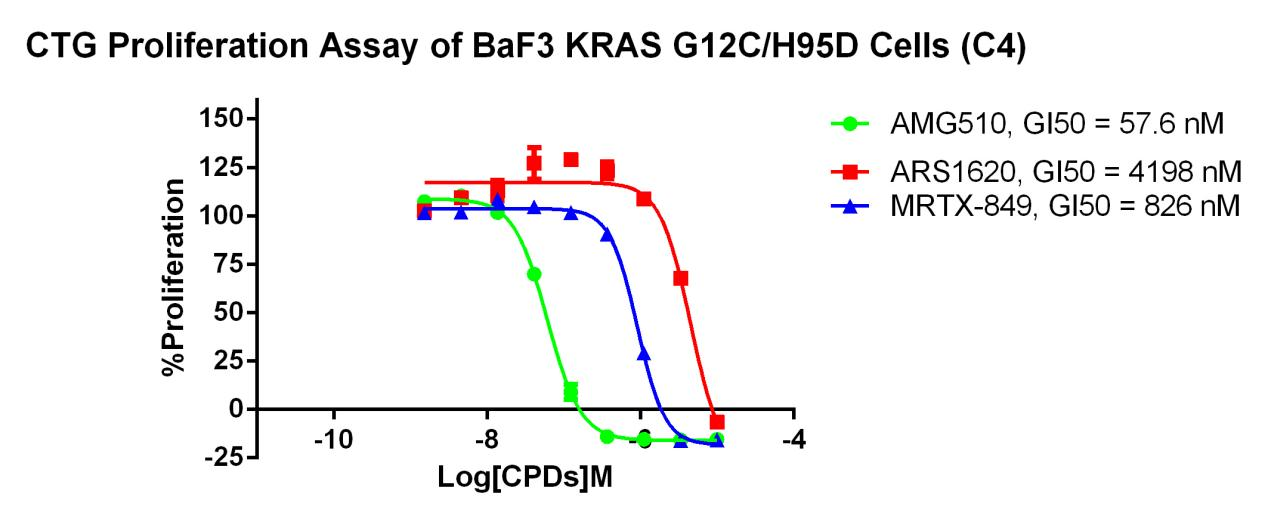
Fig 4. CTG Proliferation Assay of BaF3 KRAS G12C/H95D Cells (C4).
KRAS G12C-R68S/BaF3 RQP73344
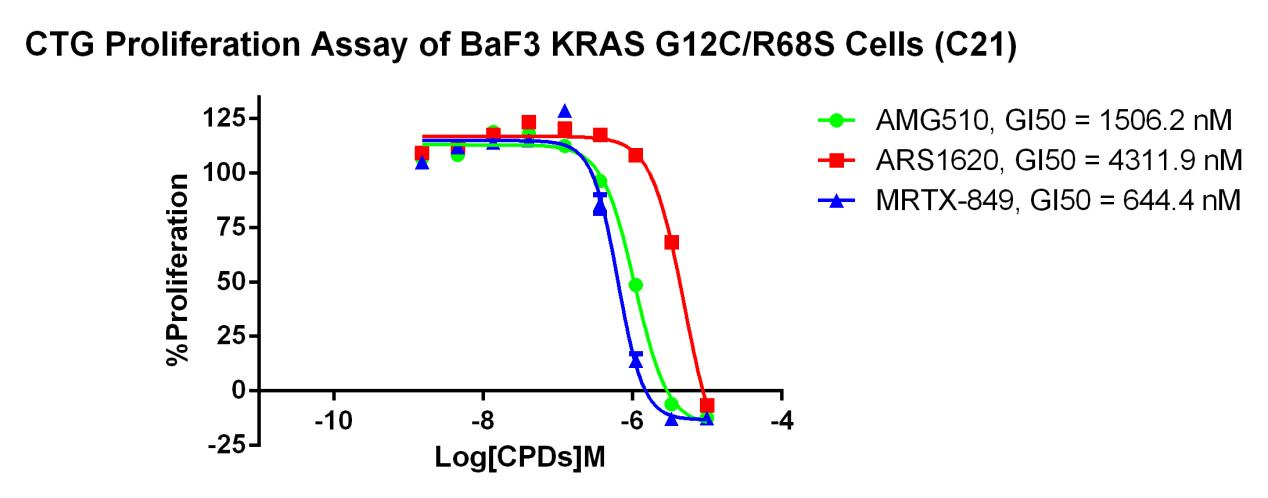
Fig 5. CTG Proliferation Assay of BaF3 KRAS G12C/R68S Cells (C21).
KRAS G12C-Y96D/BaF3 RQP73346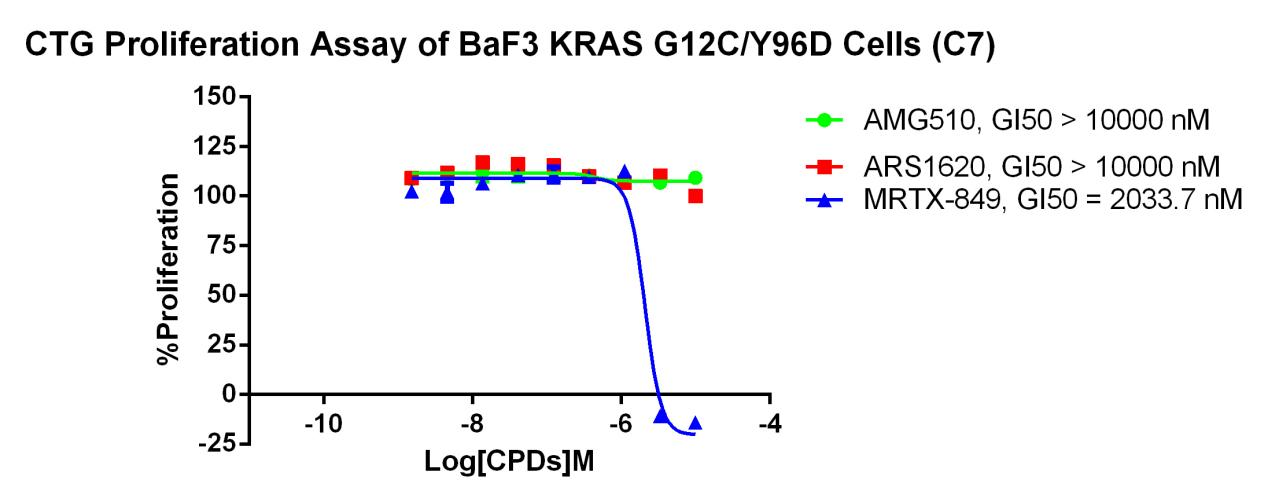
Fig 6. CTG Proliferation Assay of BaF3 KRAS G12C/Y96D Cells (C7).
KRAS G12C-Y96C/BaF3 RQP73337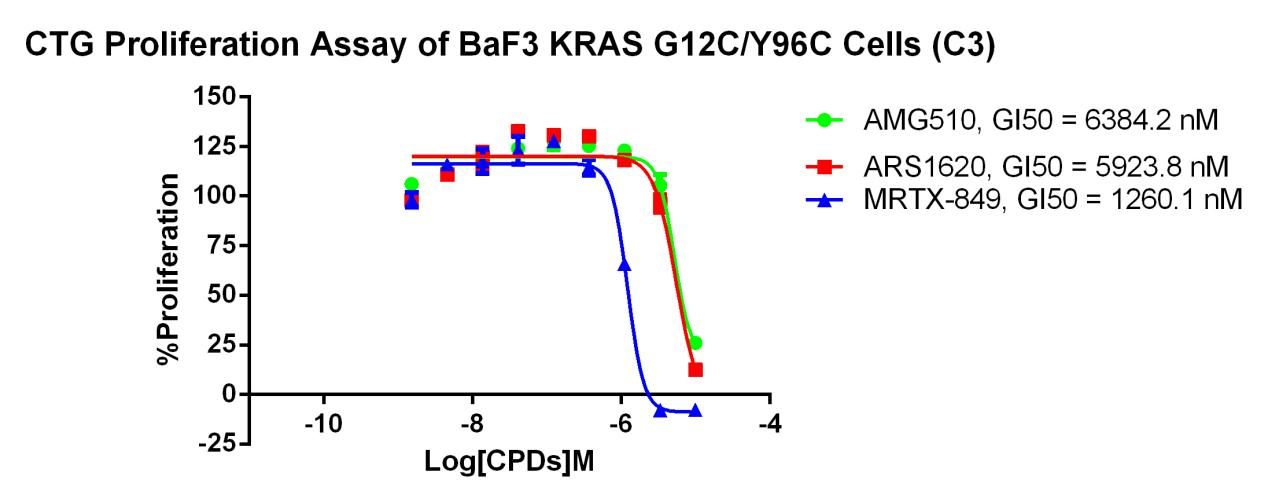
Fig 7. CTG Proliferation Assay of BaF3 KRAS G12C/Y96C Cells (C3).

