[Target model + diagnostic quality control] Drug development and diagnosis of EGFR vIII
Genetic background:
Epidermal growth factor receptor (EGFR/ErbB1/HER1) is a member of the tyrosine kinase receptor family, which also includes ErbB2/HER2/Neu, ErbB3/HER3 and ErbB4/HER4. All these receptors are transmembrane glycoproteins with molecular weights ranging from 170 to 185kDa. Generally, its activation involves ligand binding and subsequent receptor dimerization. The activation of EGF receptors can induce signals in the Ras/Raf/MAPK, PI3K/AKT, JAK/STAT or PLC/PKC pathways, and affect a variety of cellular processes, including proliferation, metabolism, apoptosis, cell survival or differentiation. The termination of the signal cascade occurs after the receptor is internalized, mainly clathrin-dependent endocytosis, leading to its entry into the early endosome. In addition, the receptor may be transported back to the cell membrane or degraded in late endosomes and lysosomes.
The gene encoding EGFR is located on the short arm of chromosome 7 (p11.2) and consists of 28 exons. The mature EGFR protein (1186 amino acids) is formed by the precursor protein (1210 amino acids) after removing the N-terminal part. From N to C-terminus, EGFR consists of an extracellular domain (exons 1-16) involved in ligand binding and receptor dimerization, a hydrophobic transmembrane domain (exon 17), and an intracellular tyrosine kinase activity. Domain composition, flanked by the linker region and the C-terminal part of the receptor (exons 18-28). Twelve of the 20 tyrosine residues in the intracellular domain have been shown to undergo phosphorylation, which binds to membrane-bound or cytoplasmic effector proteins that are recruited upon activation of receptors.
In GBM, EGFR amplification is accompanied by gene rearrangement in most cases. Such changes involve the deletion of specific exons or parts of exons, and are designated as EGFRvI (the N-terminal partial deletion), EGFRvII (the deletion of exons 14 and 15), EGFRvIII (the deletion of exons 2-7), EGFRvIV (deletion of exons 25-27) and EGFRvV (deletion of exons 25-28).
In glioblastoma (GBM), one of the most frequently detected variants is EGFRvIII. Approximately 50% of GBM patients have EGFR amplification, and approximately 50-60% of the amplification is accompanied by EGFRvIII rearrangement. The appearance of EGFRvIII is due to the deletion of EGFR exons 2-7, resulting in a truncated extracellular domain that can be constitutively activated by EGFR. The truncated extracellular domain produced a new peptide sequence, which resulted in a unique, GBM cell-specific, antibody-reactive EGFRvIII antigen.
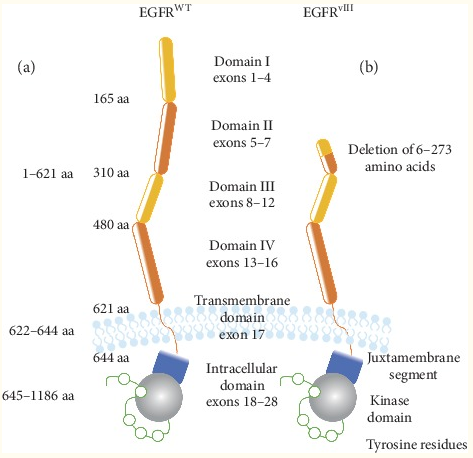
Fig 1. Schematic diagram of the structure of EGFR WT and EGFR vIII
Drug development
From a clinical point of view, glioblastoma is one of the most difficult tumors, and there is still a lack of effective treatments for patients diagnosed with this tumor type. To date, many treatment methods have been developed to treat patients with EGFR vIII-positive glioblastoma.
Current clinical and preclinical trials on EGFR/EGFR vIII therapy include small molecule tyrosine kinase inhibitors, antibodies, vaccines, and RNA interference-based therapies. Since the silencing of a single gene in a specific signaling pathway may not be sufficient to provide a therapeutic effect in GB patients, a complex method is required that focuses on several signaling pathways.
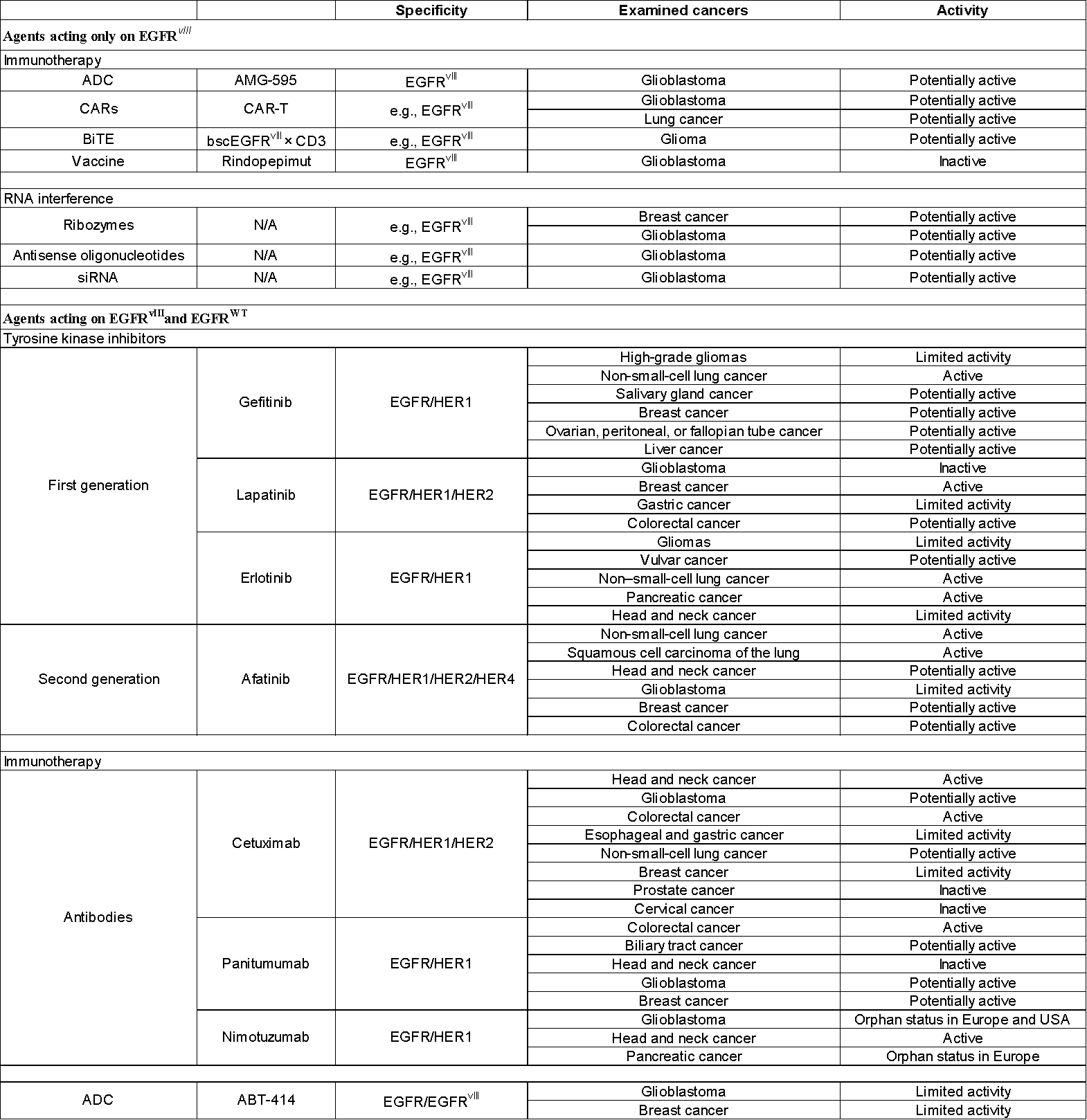
Table 1. Research therapies that target EGFR vIII alone, and target both EGFR WT and EGFR vIII
Drug target model
We develops in vitro live measurement models for EGFR WT and EGFR vIII targets, which can be used to detect the anti-tumor proliferation inhibitory activity of small molecule inhibitors.
Fig 2. EGFR WT (EGF Dependent)/BaF3 in vitro live test model, replacing IL-3 with human EGFR WT gene as the driving gene for Baf3 proliferation.

Fig 3. EGFRvIII/BaF3 in vitro live test model, using human EGFR vIII (autophosphorylation) gene to replace IL-3 as the driving gene for Baf3 proliferation.
Molecular diagnostics
In the diagnosis of GBM, the NCCN guidelines mention EGFR amplification and EGFR mutation. EGFR vIII mutations can be called EGFR mutations and often lead to EGFR amplification, so it is an important diagnostic indicator.

Fig 4. Screenshot of NCCN-2021 second edition of central nervous system tumor molecular markers
Diagnostic standards
Through gene editing, we cut genomic DNA on intron 1 and intron 7 of EGFR, and artificially edited samples of E2-E7del, which are suitable for molecular diagnostic standards at the two levels of DNA and RNA.
Fig 5. The breakpoint of EGFR vIII at the DNA level, the mutation frequency detected by ddPCR is 100%.
Fig 6. The breakpoint of EGFR vIII at the RNA level, detected by ddPCR is 6700copies/ng.

