ctDNA liquid biopsy diagnostic standard
"Liquid Biopsy" (Liquid Biopsy) refers to a technology that uses human body fluid as a specimen source to detect and obtain tumor-related information. It is very important for early diagnosis, medication monitoring, and prognosis judgment of solid tumors. It has good compliance, easy access to specimens, and specificity. Good sex and other advantages. There will be a small amount of free circulating tumor cells (CTC) in the blood of cancer patients, and the necrotic cancer cells will also release a small amount of circulating tumor DNA (ctDNA) into the blood, so it can be detected from the primary tumor Or CTC and ctDNA released into the blood from the metastatic site to detect cancer. ctDNA (circulating tumor DNA), or circulating tumor DNA, refers to DNA fragments in the human blood that carry tumor-specific gene mutations, deletions, insertions, rearrangements, copy number variations, and methylation.
Detection method
Commonly used ctDNA detection techniques in laboratories include amplification refractory mutation system (ARMS), next-generation sequencing (NGS), digital PCR (digital PCR, dPCR) and nucleic acid mass spectrometry. When detecting known, single targeted therapy sensitive or resistant mutations, it is recommended to use the ARMS method; when detecting known, multiple parallel clinical therapeutic targets or discovering unknown genes, and exploring the clinical value and related mechanisms, it is recommended to use the NGS method; dPCR It has the potential to verify NGS test results, but its test performance and clinical significance should be strictly evaluated before application.
Early tumor diagnosis
Clinical application guidance
Targeted drug companion diagnosis
Real-time efficacy monitoring
Early warning of tumor progression and poor prognosis
Probing into the mechanism of drug resistance
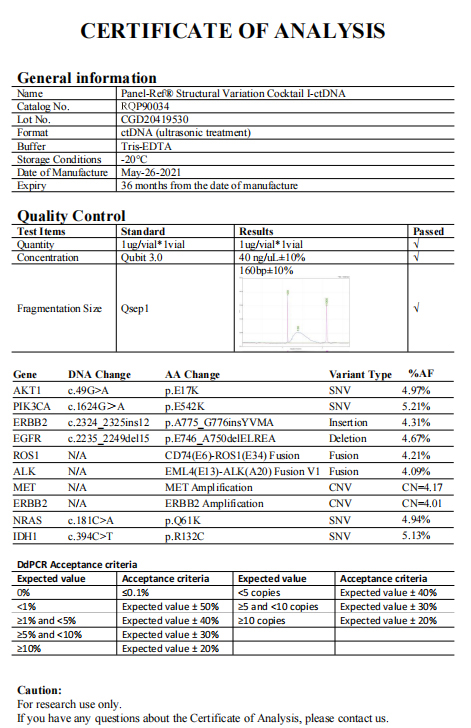
In view of the important application of ctDNA detection in tumor diagnosis and treatment, the detection process also requires full quality control. We can provide our customers with ctDNA diagnostic standard products, which are highly simulated clinical samples by means of enzyme digestion or ultrasound, and are accurately calibrated by DdPCR, which can be applied to the entire process of detection on each platform.
Part of the product data
Panel-Ref® Structural Variation Cocktail I-ctDNA RQP90034