[Product Promotion] VEGFR-2 (KDR) Small Molecule Inhibitor Cell Screening Model
Vascular endothelial growth factor receptor (VEGFR) belongs to the superfamily of tyrosine kinase receptors, including VEGFR-1, VEGFR-2 (also known as KDR) and VEGFR-3. Among them, VEGFR-1 is mainly expressed in the development of hematopoietic cells, VEGFR-3 can promote the growth of lymphatic endothelial cells, and VEGFR-2 is mainly expressed in vascular endothelial cells and hematopoietic stem cells, which can mediate cell signal transduction pathways regulated by VEGF , Stimulate the proliferation of vascular endothelial cells, leading to the growth of blood vessels. Studies have shown that VEGFR-2 is highly expressed in a variety of malignant tumors, including ovarian cancer, thyroid cancer, melanoma and medulloblastoma. Therefore, in the past ten years, VEGFR-2 has become an important target for the development of tumor anti-angiogenesis drugs, and many drugs have been on the market.
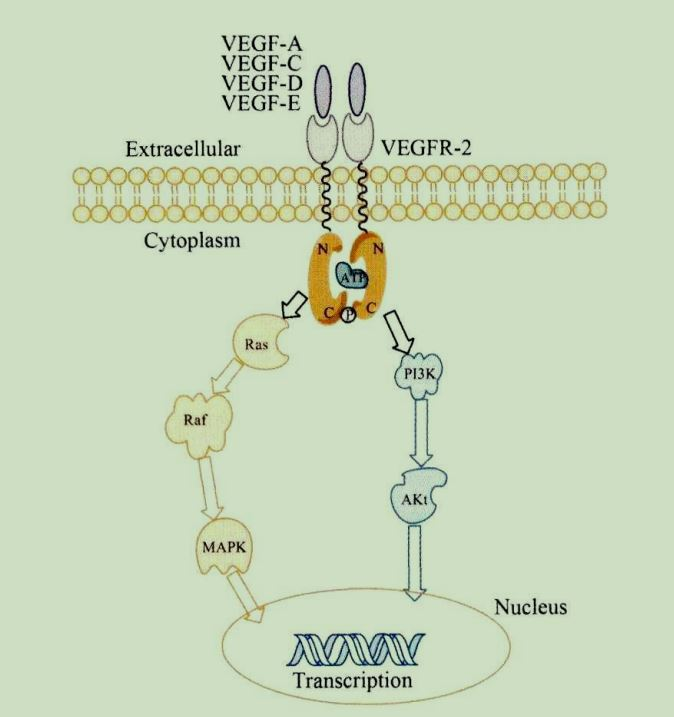
VEGFR-2 can bind to a variety of VEGF ligands (VEGF-A, C, D, E) to form a homodimer complex, which changes the protein conformation, and autophosphorylates tyrosine residues. The signal is transmitted downstream.
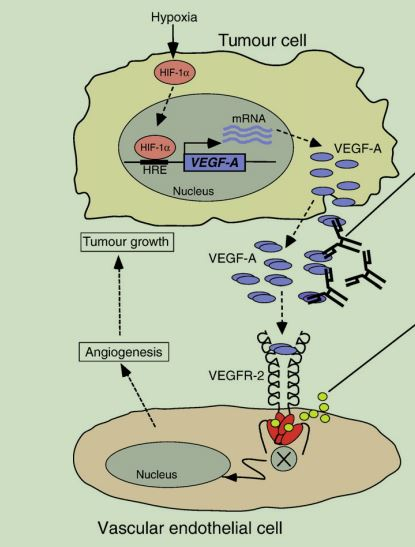
Among them, the main signal pathways include Ras/Raf/MAPK and PI3K/AKT. These signal pathways will activate transcription factors, interfere with the cell cycle, promote cell migration, maintain cell proliferation, inhibit cell apoptosis, and promote tumorigenesis and develop. On the other hand, growing tumor cells can activate HIF-1α protein under hypoxia, induce the transcriptional expression and secretion of VEGF-A, and bind to VEGFR-2 on vascular endothelial cells around tumor cells to stimulate angiogenesis. Causes tumor growth. Therefore, it is possible to inhibit VEGFR-2 signal transduction through antibody binding to VEGF-A or VEGFR-2 protein, or to inhibit VEGFR-2 receptor kinase activity through small molecule inhibitors, so as to obtain therapeutic effects.
Research and development status of VEGFR-2 (KDR) small molecule inhibitors
Because VEGFR-1, 2, 3 have similar structures and have high catalytic region homology with PDGFR, c-kit, CSF1R, FLT3 and other kinases, VEGFR-2 small molecule inhibitors are generally multi-target inhibitors Inhibition of VEGFR-2 can reduce angiogenesis, inhibition of VEGFR-3 can reduce tumor lymphatic spread, inhibition of PDGFR, c-kit, CSF1R, FLT3 can block important signal pathways for tumor growth, and there have been many small VEGFR-2 inhibitors The drug has been approved for marketing or is in the clinical research stage:
(1) Sunitinib, developed by Pfizer, was approved by the FDA in 2006 for the treatment of advanced renal cell carcinoma (RCC), gastrointestinal stromal tumor (GIST), and metastatic well-differentiated advanced pancreatic neuroendocrine tumor ( pNET).
(2) Vandetanib, developed by AstraZeneca, was approved by the FDA in 2011 for the treatment of patients with medullary thyroid cancer that cannot be surgically removed or continues to spread to other parts of the body.
(3) Ninetdanib, developed by Boehringer Ingelheim, was approved by the FDA for the first time in 2014 for the treatment of patients with idiopathic pulmonary fibrosis (IPF). In 2019, it was approved by the FDA for the alleviation of patients with systemic sclerosis-related interstitial lung disease (SSc-ILD). In 2020, it was approved by the FDA for chronic fibrotic interstitial lung disease (PF-ILD).
(4) Lenvatinib developed by Eisai is a multi-target kinase inhibitor against vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3, including FGFR1, 2, 3, 4, PDGFRα and RET. It was approved by the FDA in 2015 for thyroid cancer, in 2016 for advanced renal cell carcinoma, and in 2018 as a first-line treatment for unresectable hepatocellular carcinoma (HCC).
VEGFR-2 (KDR) small molecule inhibitor cell screening model
TEL-KDR/BaF3 CBP73305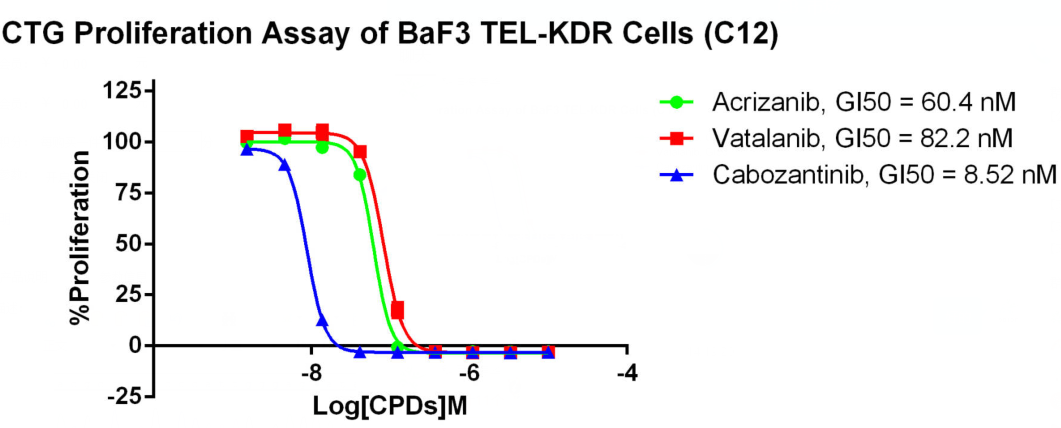
Figure 3. CTG Proliferation Assay of BaF3 TEL-KDR Cells (C12).

