PVRIG CD112 drug target screening model
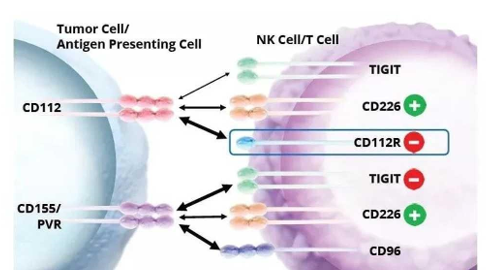
Human PVRIG (poliovirus receptor-related immunoglobin domain-containing protein), also known as CD112 receptor (CD112R), is a poliovirus receptor-like protein (poliovirus receptor-like protein). like protein, PVR) is a transmembrane protein of about 34kda in the family. The gene encodes a transmembrane protein consisting of an extracellular IgV domain, a transmembrane domain, and a long intracellular domain. PVRIG (CD112R) is mainly expressed on T cells and NK cells, and regulates immune function. When PVRIG is combined with its ligand PVRL2 (CD112, Nectin-2) on antigen presenting cells, it will inhibit immune activation.
In addition, PVRIG can also exert an immunosuppressive effect by binding to its ligand competitively with CD226 to block the stimulus signal it transmits. This is similar to the immunosuppressive mechanism of TIGIT, a member of the same family, and the two may have a synergistic effect on immunosuppression. (As shown in Figure 1) It has been found in clinical practice that PVRL2 is overexpressed in a variety of tumor types including breast and ovarian cancer, indicating that its interaction with PVRIG may be a major mechanism of immunosuppression in these diseases.
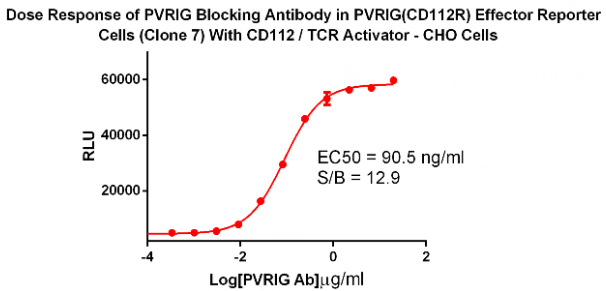
Preclinical data shows that blocking the interaction of PVRIG and CD112 with antibodies can enhance the killing effect of T cells; at the same time, experiments have also shown that blocking PVRIG and TIGIT can enhance the killing effect of Trastuzumab-mediated NK cells on breast cancer. Based on some of the above experimental data, PVRIG has been considered as a new and important immune checkpoint therapeutic target, and a new generation of antibody drugs will be developed for it.
At present, some companies have made certain progress in research and development. For example, as early as 2018, Israel Compugen announced that its therapeutic antibody COM701 against PVRIG was approved by the FDA to enter clinical trials for the treatment of malignant solid tumors. This is the first approval in the world. In the same year, the company also announced a cooperation with Bristol-Myers Squibb (BMS) to evaluate the safety and tolerability of the antibody in combination with BMS's Opdivo (nivolumab) in the treatment of advanced solid tumors. In the future, COM701 will be combined with BMS's PD-1 antibody (Opdivo®) and TIGIT antibody (BMS-986207) in clinical trials in the treatment of advanced solid tumors.
In addition, in 2020, GSK spent more than US$800 million to reach a cooperation agreement with Surface Oncology to introduce its global development and commercialization rights for the PVRIG antibody SRF831, which is still in the preclinical stage.
Aiming at this important emerging immune checkpoint target, Kebai Biological has developed a related cell screening model, which can be used for functional screening and live measurement.
PVRIG(CD112R) Effector Reporter Cell RQP74120
CD112/TCR Activator/CHO RQP74121