AKT E17K mutation inhibitor cell screening model
The AKT series of proteins are serine/threonine protein kinases, which can be activated by extracellular signals through a phosphatidylinositol 3-kinase (PI3K)-dependent mechanism. There are currently three members of the AKT family, namely AKT1, AKT2 and AKT3. The significant mutations in tumors are mainly point mutations in the kinase domains of E17K and Q79K.
The AKT series of proteins are the core factors in the PI3K/AKT signaling pathway. PI3Ks can specifically phosphorylate phosphatidylinositol (PI) to produce the second messenger inositol PIP3, which can promote the transfer of AKT to the cell membrane and be PDK1/PDK2 Activation, activated AKT relocates to the cytoplasm, nucleus or other parts of the cell, phosphorylates a large number of substrate proteins, and then regulates cell function.
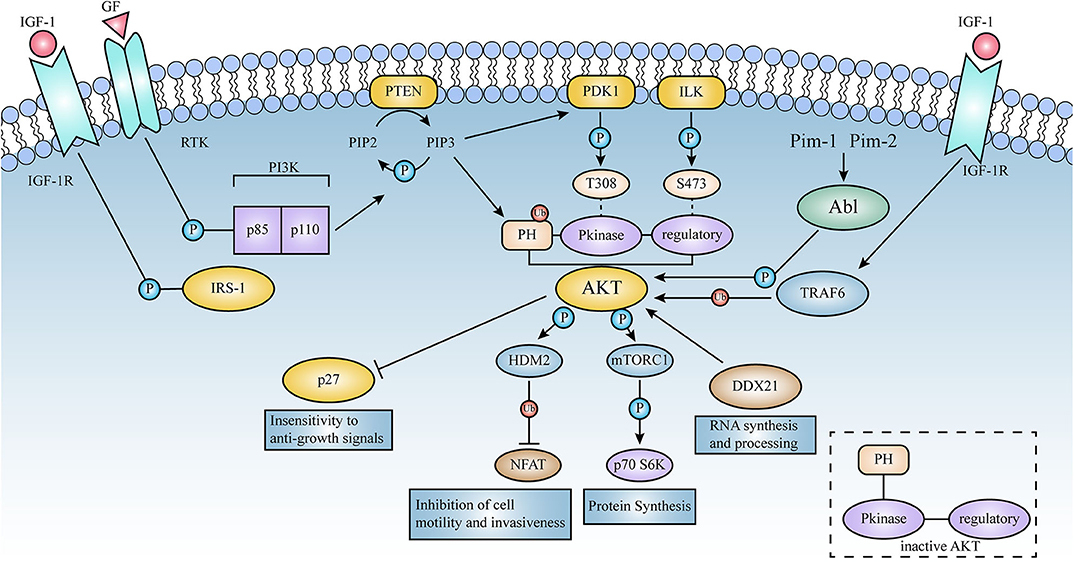
Mutations of AKT protein, especially E17K mutations can cause abnormal activation of ATK protein, which plays an important role in tumor occurrence, development, metastasis and drug resistance. Current studies have found that abnormal activation of ATK has the following three effects on tumors:
1. Promote cell production and value-added, causing cells to transform from benign to malignant;
2. Promote cell movement and invasion, causing tumor metastasis and dissemination;
3. Inhibit cell apoptosis and promote blood vessel growth, and promote tumor blood vessel growth;
4. Related to chemotherapy resistance/endocrine resistance, leading to failure of other drug treatments.
For the AKT target, no product has been approved for marketing at home and abroad so far, but many domestic and foreign pharmaceutical companies have deployed AKT inhibitors, and drugs have entered phase 3 clinical trials with positive results.
1. Ipatasertib is a pan-Akt competitive inhibitor, jointly developed by Roche and ArrayBioPharma. Phase III clinical trials are currently underway for metastatic castration-resistant prostate cancer and triple-negative breast cancer. Among them, the results of phase III clinical trials for the treatment of metastatic castration-resistant prostate cancer have been announced. Compared with the Zytiga single-agent group, the combination therapy did not achieve statistically significant clinical benefit improvement, but in patients with PTEN gene deletion, the combination therapy improved The patient's progression-free survival (PFS, 6.2 months vs 3.7 months). The results of a phase II study of triple-negative breast cancer showed that paclitaxel combined with oral AKT inhibitor ipatasertib can prolong the overall survival rate of patients with locally advanced or metastatic triple-negative breast cancer (25.8 months vs 16.9 months).
2. Capivasertib is a new type of AKT1/AKT2/AKT3 inhibitor developed by AstraZeneca. It is currently conducting Phase III clinical trials for triple-negative breast cancer and prostate cancer. Previously published Phase II data showed that for patients with PIK3CA/AKT1/PTEN mutations, paclitaxel + Capivasertib had a better effect. The median PFS was prolonged by 5.6 months, and the median duration of remission was prolonged by 9.8 months. In addition, Capivasertib combined with fulvestrant in the treatment of estrogen receptor-positive metastatic breast cancer after the progress of endocrine therapy also obtained positive results, and fulvestrant + Capivasertib can significantly prolong the median PFS (10.3 months vs 4.8 Month).
In response to the development and research needs of AKT E17K mutation inhibitors, we have developed AKT1 E17K/BAF3, AKT2 E17K/BAF3 and AKT3 E17K/BAF3 drug screening cell lines. Welcome to inquire.
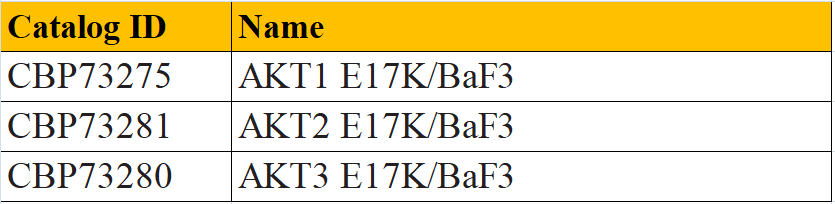
AKT1 E17K/BaF3 CBP73275 Figure 3. CTG Proliferation Assay of BaF3 AKT1 E17K Cells (C3). AKT2 E17K/BaF3 CBP73281 Figure 4. CTG Proliferation Assay of BaF3 AKT2 E17K Cells (C4). AKT3 E17K/BaF3 CBP73280 Figure 5. CTG Proliferation Assay of BaF3 AKT3 E17K Cells (C2).

