BTLA cell screening model
BTLA is a glycoprotein containing immunoglobulin domains, expressed on T cells, dormant B cells, macrophages, dendritic cells and a small number of NK cells. BTLA is an inhibitory receptor of T cells. Anti-BTLA treatment can lead to the proliferation of T cells. BTLA knockout mice show highly reactive immune activation. HVEM is a tumor necrosis factor receptor, which has been identified as a natural ligand for BTLA in mice and humans. Antigen-presenting cells (APCs) expressing HVEM can induce BTLA-dependent T cell suppression.
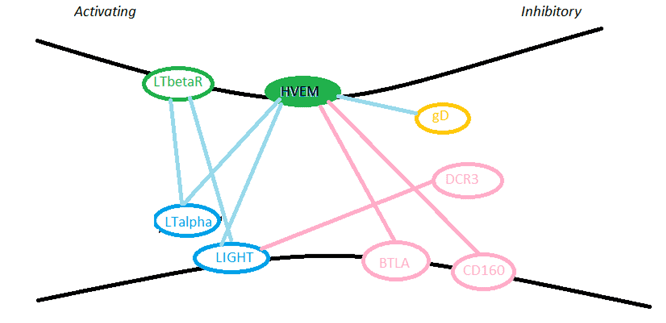
BTLA, CTLA-4 and PD-1 belong to the immunoglobulin superfamily, in which CTLA-4 and PD-1 bind to members of the B7 family. While HVEM is a member of the tumor necrosis factor receptor family, the interaction of BTLA/HVEM proved for the first time that there is signal crossover between these two receptor families. Before the discovery of the BTLA/HVEM interaction, HVEM has been found to bind to the tumor necrosis factor ligand LIGHT and lymphotoxin-α. The interaction of BTLA/HVEM generates co-inhibition signals, and the interaction of HVEM/LIGHT generates co-stimulatory signals through HVEM. (As shown in Figure 1)
The BTLA/HVEM interaction plays a key role in the regulation of inflammation, autoimmunity and anti-tumor response. In patients with melanoma, BTLA is expressed on tumor-specific T cells in circulating and metastatic lymph nodes. HVEM is highly expressed in most melanoma cell lines and metastatic melanoma samples. The interaction between BTLA-expressing tumor-specific T cells and HVEM-positive melanoma cells results in T cell suppression, and BTLA blocking antibodies can reverse this suppression. In an in vitro test of cells derived from melanoma patients, it was found that the joint blockade of BTLA and PD-1 pathways significantly increased the number and function of tumor-specific killer lymphocytes, which was much better than the blocking effect of PD-1 alone. It shows that BTLA can be used as an important target for the development of tumor immunotherapy drugs.
At present, this target has been paid attention to and developed by some new drug research and development companies. In March 2019, Junshi Bio announced the development of the world's first recombinant humanized IgG4κ monoclonal antibody JS004 specifically for BTLA, which was accepted by the FDA for late stage Clinical trials for the treatment of unresectable or metastatic solid tumors (including lymphoma), 2020 The antibody has also been approved for clinical trials in China, and is currently in clinical trials at home and abroad.
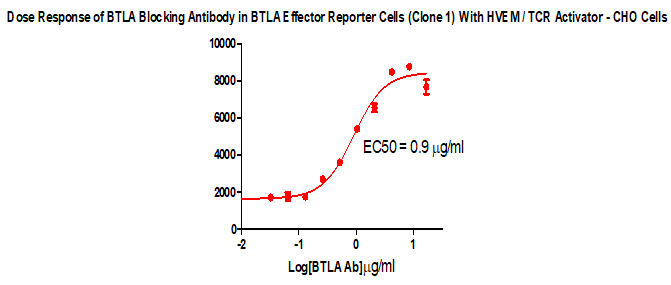
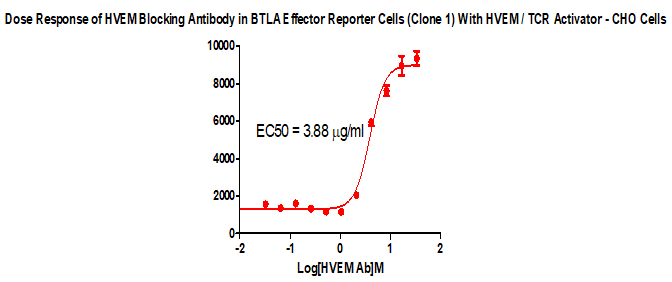
For the development of antibody drugs that inhibit the BTLA/HVEM signaling pathway, we have developed an in vitro screening cell model that can be used to screen the functional activity of blocking antibodies against BTLA/HVEM binding.
BTLA Effector Reporter Cell RQP74118
HVEM/TCR Activator/CHO RQP74082