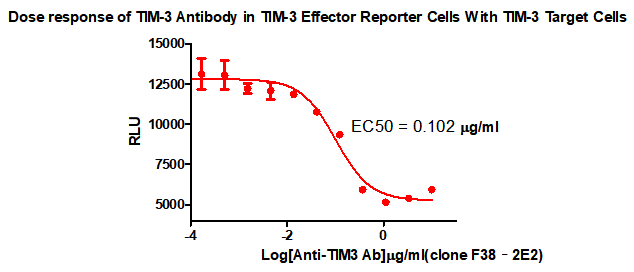
TIM-3 cell screening model
TIM-3 (CD366, HAVCR2) is an immune checkpoint receptor, often expressed on certain activated T cell subsets, regulatory T cells (Tregs), macrophages and dendritic cells. Preclinical studies have shown that TIM-3 signal regulates Th1 cytokine response, and its expression is related to autoimmune resistance.
Although the in vivo effects of TIM-3 are clear, the mechanism leading to these results at the cellular level is unclear. More and more literatures show that the role of TIM-3 in T cell activation due to different environments and cell types is more complicated than previously reported. In different in vitro systems, TIM-3 has the ability to inhibit or co-stimulate T cell receptor signal transduction. In addition, the determination of TIM-3 ligand and its related functions has long been a topic of intense debate in the scientific community. Phosphatidylserine (PS), galectin-9, CEACAM-1 and other ligands considered to be TIM-3 add another layer of complexity to the study of TIM-3 signaling pathway.
One obvious difference between TIM3 and other immune checkpoint molecules is that it is not up-regulated after all T cells are activated. It is only up-regulated in CD4+ helper T cell 1 (Th1) and CD8+ cytotoxic T cells. It participates in synergistic inhibition. After TIM3 is activated It will inhibit the activity of effector T cells and cause peripheral tolerance. TIM3 plays a key role in the depletion of T cells in tumors. Studies have found that blocking TIM-3 can lead to resistance to tumor growth in mouse models of colorectal cancer and ovarian cancer; in addition, TIM3 is highly expressed in T cells in anti-PD-1 resistant animal models, while anti-TIM3 antibodies are associated with anti-PD -1 drug combination can inhibit the development of resistance to anti-PD-1 therapy.
At present, many domestic and foreign pharmaceutical companies are actively deploying the development of TIM-3 antibody drugs. Abroad, GlaxoSmithKline’s TSR-022, Novartis’ MBG453 and Bristol-Myers Squibb’s BMS-986258 have all entered the phase II clinical phase; domestic pharmaceutical companies, Hengrui Medicine SHR-1702, KL-Biotech KL- A293, BeiGene BGB-A425 has also entered the clinical trial application stage. The following table lists some TIM-3 drugs under development, including single drugs or research progress in combination with PD-1/PD-L1 antibodies.
To this end, we have also developed a cell screening model for the TIM-3 target for in vitro detection of TIM-3 antibody drugs for functional screening.