[Serial-Corporate Reference] Selection of Reference Materials for Diagnosis of Gastric Cancer
As a company that provides enterprise nucleic acid diagnostic standard products, Kebai has been deeply involved in this field for many years. It has cooperated with 90% of enterprises in this field in the country, and has successfully supported the development and approval of kits of many enterprises. with rich experience. According to our market research and understanding of customer needs, we divide our product line into multiple modules, mainly single-point mutation, fusion and CNV products that support PCR, and small panels and large panels that support NGS. , Including TMB and MSI) products, FFPE block products that support IHC and FISH, and dPCR test kit products that support standard product calibration.
In the next few issues, we will follow the NCCN guidelines for unused tumors and share our views on the selection of corporate reference products.
Gastric carcinoma is a malignant tumor that originates from the epithelium of the gastric mucosa. It ranks first among all kinds of malignant tumors in my country. There are obvious regional differences in the incidence of gastric cancer. The incidence of gastric cancer in the northwest and east coastal areas of my country is higher than that in the south. The area is obviously high. The prevalent age is over 50 years old, and the ratio of male to female incidence is 2:1. Due to changes in diet, increased work pressure, and Helicobacter pylori infection, gastric cancer tends to be younger. Gastric cancer can occur in any part of the stomach, and more than half of them occur in the antrum. The greater curvature of the stomach, the lesser curvature, and the front and back walls can be affected. The vast majority of gastric cancer is adenocarcinoma. There are no obvious symptoms in the early stage, or non-specific symptoms such as upper abdominal discomfort, belching, etc., which are often similar to the symptoms of chronic gastric diseases such as gastritis and gastric ulcer, and are easily ignored. Therefore, the current early diagnosis of gastric cancer in my country The rate is still low. The prognosis of gastric cancer is related to the pathological stage, location, tissue type, biological behavior and treatment measures of gastric cancer.
For the diagnosis of gastric cancer, the molecular markers written into the NCCN and expert consensus are mainly HER2 (ERBB2) detection, microsatellite instability (MSI) and DNA mismatch repair deficiency (dMMR) detection, PD-L1 detection, as well as NTRK-fusion detection.
For HER2, IHC is recommended to detect protein expression. Fish's method is used to detect CNV. NGS can also be used. In addition to HER2, other molecular markers can also be detected.
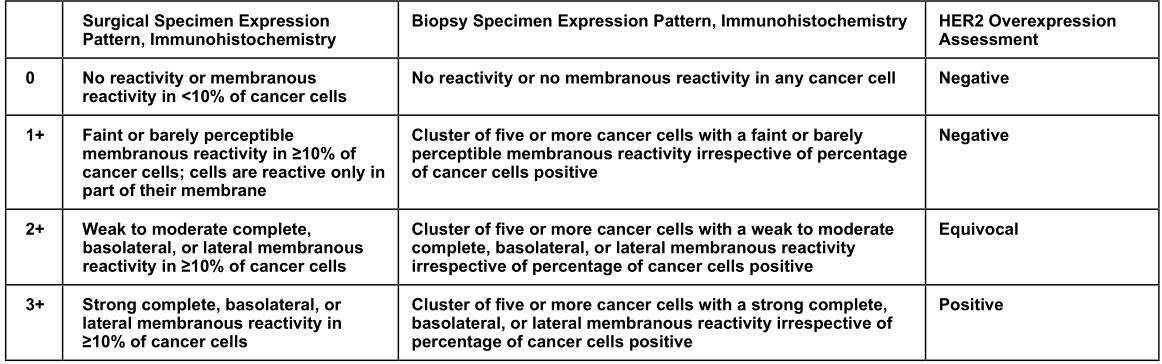
For the IHC standard product of HER2 protein, Kebai Bio is under development;
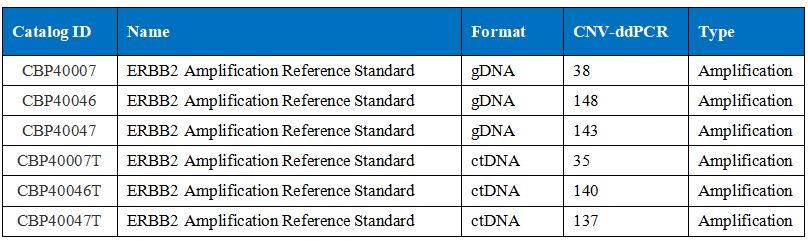
For the molecular standard of HER2 CNV, Cobai mainly uses the ddPCR method to calibrate the CNV of HER2. The method can refer to: 2016 NSIT standard 2373.
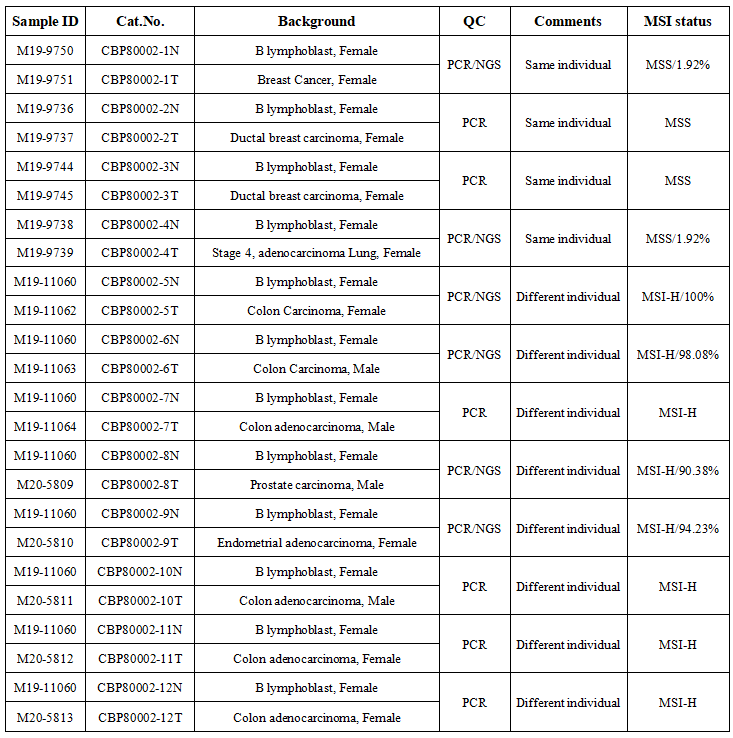
MSI/MMR
An analysis of 8 studies and 1976 patients with gastric cancer showed that the ratio of MSI-H patients was 11.68%-33.82%. Four studies used NCI standards to define MSI-H, and 3 studies suggested that MSI-H gastric cancer tended to Intestinal gastric cancer has a better prognosis. A clinical study reported that in MSI-H/dMMR gastric cancer, compared with patients with surgery alone, the prognosis of patients receiving preoperative chemotherapy plus surgery is poor, suggesting that MSI/MMR status testing may help screen patients with gastric cancer who need preoperative chemotherapy.
Kebai Gene has launched a number of MSI test standards:
PD-L1 Detect
According to the NCCN guidelines, PD-L1 detection can be performed for patients with locally advanced, recurrent or metastatic gastric cancer to evaluate the expression of PD-L1 protein in gastric cancer. This is a qualitative immunohistochemical assay using anti-PD-L1 antibody to detect PD-L1 protein in FFPE tissue of gastric adenocarcinoma. At least 100 tumor cells must be present on the PD-L1 stained slide for the specimen to be considered sufficient for PD-L1 evaluation. If the comprehensive positive score (CPS) ≥ 1, the sample is considered to have PD-L1 expression. CPS is the number of PD-L1 stained cells (ie, tumor cells, lymphocytes, macrophages) divided by the total number of surviving tumor cells, and then multiplied by 100.
The IHC standard product of PD-L1 is being developed by Kebai Bio, and it is expected to be launched in 2021.
NTRK Fusion
In addition, the FDA has also approved TRK inhibitors to treat a variety of solid tumors in TRK-fusion, and Kebai provides a variety of NTRK-Fusion RNA standards.
summary
In summary, in accordance with NCCN guidelines, clinical drugs, and companion diagnostic requirements, Kebai Biosciences has developed various reference products and standard products for Thyroid Carcinoma, which are suitable for different applications and meet the needs of different customers. .
After selecting the standard products, the next step is to perform performance evaluation. Let us first learn the guiding principles and regulations for performance evaluation.
For details, please refer to "Analysis of Reference Design from the "Guiding Principles"."

