Selection of diagnostic reference materials for acute lymphoblastic leukemia
As a company that provides enterprise nucleic acid diagnostic standard products, Reqbio has been deeply involved in this field for many years. It has cooperated with 90% of enterprises in this field in the country, and has successfully supported the development and approval of kits of many enterprises. extremely experienced. According to our market research and understanding of customer needs, we divide our product line into multiple modules, mainly single-point mutation, fusion and CNV products that support PCR, and small panels and large panels that support NGS. , Including TMB and MSI) products, FFPE block products that support IHC and Fish, and dPCR detection kit products that support standard product calibration.
In the next few issues, we will follow the NCCN guidelines for unused tumors and share our views on the selection of corporate reference products.
Acute lymphocytic leukemia (ALL) is a malignant tumor disease in which B-line or T-line cells derived from lymphocytes proliferate abnormally in the bone marrow. Abnormally proliferated primitive cells can accumulate in the bone marrow and inhibit normal hematopoietic function. At the same time, it can invade tissues outside the bone marrow, such as meninges, lymph nodes, gonads, and liver. my country has conducted a survey on the incidence of leukemia, and the incidence of ALL is about 0.67 per 100,000. The incidence rate in oil fields and contaminated areas is significantly higher than the national incidence rate. ALL childhood (0-9 years old) is the peak incidence, which can account for more than 70% of childhood leukemia. ALL accounts for about 20% of adult leukemias in adults. At present, the corresponding treatment plan based on the different biological characteristics of ALL has achieved good results. About 80% of children and 30% of adults can achieve long-term disease-free survival and have the possibility of curing.
According to the classification of WHO in 2016, ALL is mainly divided into:

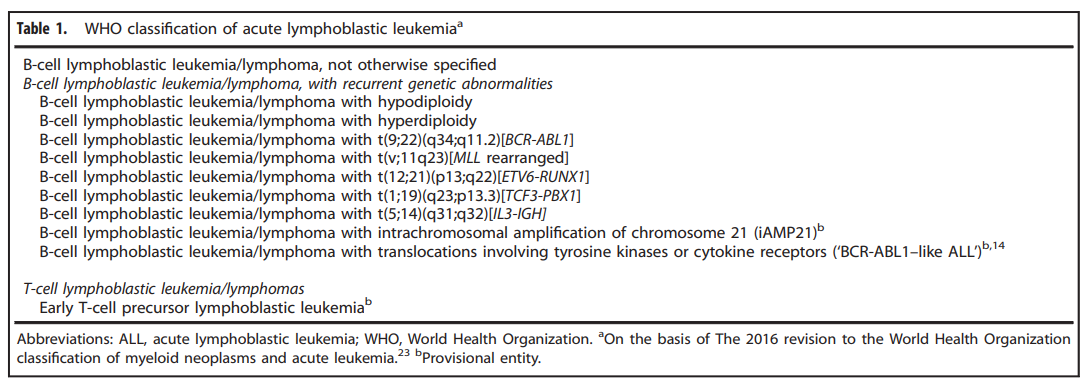
At the same time, his proportion in the crowd is:
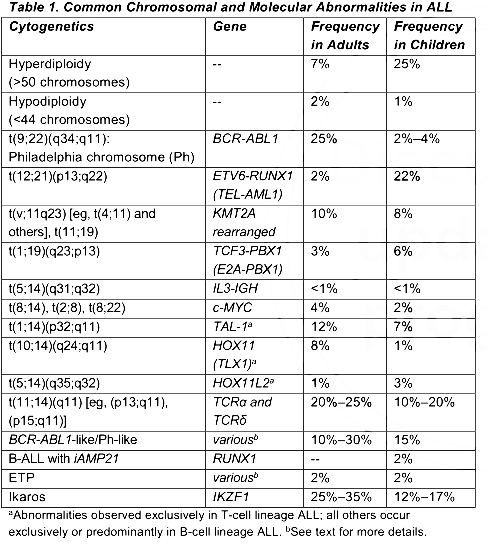

ccording to the recommendations of the NCCN guidelines, the main relevant molecular markers are:
1. Abnormality of chromosome ploidy;
2. Fusion of BCR-ABL1, mainly P190, P210 and P230;
3. Molecular rearrangement of KMT2A;
4. ETV6-RUNX1(TEL-AML1)的Fusion;
5. TCF3(E2A)-PBX1的Fusion;
6. IL3-IGH的Fusion;
7. iAMP21: intrachromosomal amplification of chromosome 21;
8. Ph-like: associated with recurrent gene fusions and mutations that activate tyrosine kinase pathways and includes gene fusions involving ABL1,ABL2, CRLF2, CSF1R, EPOR, JAK2, or PDGFRB and mutations involving FLT3, IL7R, SH2B3, JAK1, JAK3, and JAK2 (in combination with CRLF2 gene fusions).
Chromosome ploidy abnormalities
The abnormalities of chromosome ploidy are mainly divided into: Hypodiploidy (<44 chromosomes) and Hyperdiploidy (>50 chromosomes). Kebai provides all standard products of Hyperdiploidy abnormal karyotype.
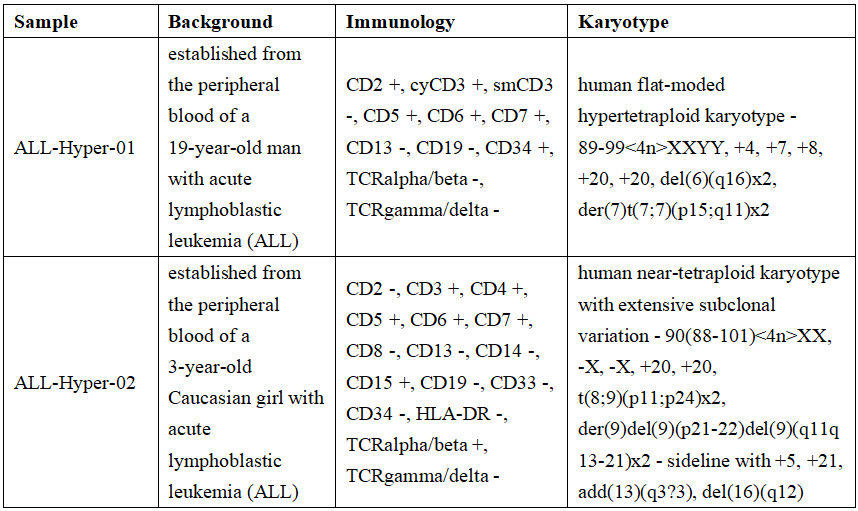

Fusion of BCR-ABL1
Ph chromosome translocation (9;22)(q34;q11) leads to BCR-ABL1 Fusion. This fusion mutation is very important. It can be found in almost all patients with chronic myeloid leukemia. In ALL, from children 5-10% to 20-30% of adults, BCR-ABL1 fusion mainly has three subtypes of P190, P210 and P230, mainly the first two; in adult patients, about 50-70% are P190 (e1a2/e3a2 ), about 15-30% of patients are P210 (b2a2/b3a2), in children, up to 90% are P190.
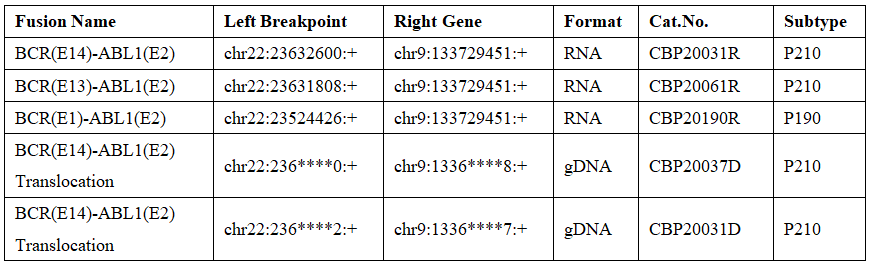
Reqbio provides different types of BCR-ABL1 fusion standard products, which can be divided into DNA and RNA forms.

Molecular rearrangement of KMT2A
The KMT2A gene, also known as MLL, is located at 11q23.3, has 21 exons and 431KDa protein. The rearrangement of KMT2A is about 4%-8% in adult patients, higher in children, and higher in infants. Sometimes it can reach 80%; in addition, it has been found in the clinic that he has many translocation partners, the most important of which are MLLT3, MLLT3, MLLT1 (ENL), ELL, EEN, CREBBP, AFF1 and so on.
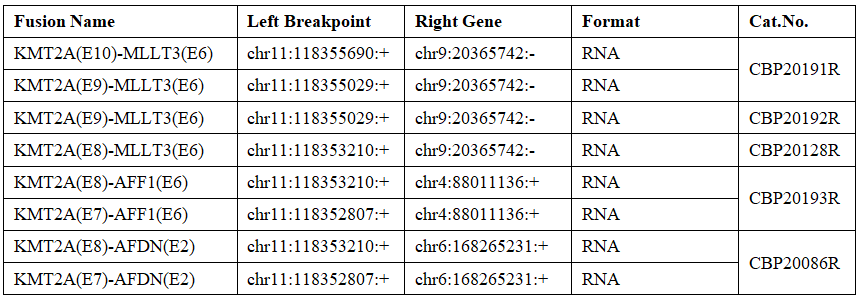

ETV6-RUNX1 Fusion
The t(12; 21) translocation combines the protein dimerization domain of ETV6 with all the DNA binding and activation regions of RUNX1. The latter is a key component of the core binding factor complex (ie core binding factor-α/RUNX1) and is essential for normal hematopoiesis.
ETV6-RUNX1 Fusion is the most common chromosomal lesion in childhood B-cell precursor acute lymphoblastic leukemia (ALL), with a total incidence of 25%.


TCF3-PBX1 Fusion
The translocation t(1; 19) (q23; p13) that causes the TCF3-PBX1 fusion gene is a chromosomal abnormality of pre-B ALL in children, accounting for 3-5% of all ALL. Historically, TCF3-PBX1 positive leukemia is associated with poor prognosis, but it has lost its prognostic significance in modern ALL chemotherapy, especially ALL chemotherapy containing high-dose methotrexate.


IL3-IGH Fusion
IGH-IL fusion in translocation t(5; 14) (q31; q32) is recognized by the WHO as a hematological malignancy with eosinophilia, B-ALL. The translocation t(5; 14) (q31; q32) juxtaposes the IGH enhancer at 14q32 with the IL3 gene at 5q31. The subsequent production of interleukin 3 (IL3) induces the maturation and release of eosinophils.
iAMP21
The intrachromosomal amplification of chromosome 21 (iAMP21) is produced by the break-fusion-bridge cycle, and chromosome necrosis is an obvious sign of the subgroup of B-cell acute lymphoblastic leukemia (B-ALL) cases and is associated with a poor prognosis. iAMP21 accounts for 2% of pediatric B-ALL, mainly in older children or adolescents.
Other
In addition, in the NCCN guidelines, some other genes related to tyrosine kinase activation are also mentioned, which can also be used as markers for diagnosis (associated with recurrent gene fusions and mutations that activate tyrosine kinase pathways and includes gene fusions involving ABL1, ABL2, CRLF2 , CSF1R, EPOR, JAK2, or PDGFRB and mutations involving FLT3, IL7R, SH2B3, JAK1, JAK3, and JAK2 (in combination with CRLF2 gene fusions). These genes do not have a significant clinically high ratio and are more suitable for panel detection.

summary
In summary, in accordance with NCCN guidelines, clinical drugs, and companion diagnostic requirements, Kebai has developed various reference products and standard products for acute lymphoblastic leukemia (ALL), which are suitable for different applications and meet the needs of different customers. demand.
After selecting the standard products, the next step is to perform performance evaluation. Let us first learn the guiding principles and regulations for performance evaluation.

